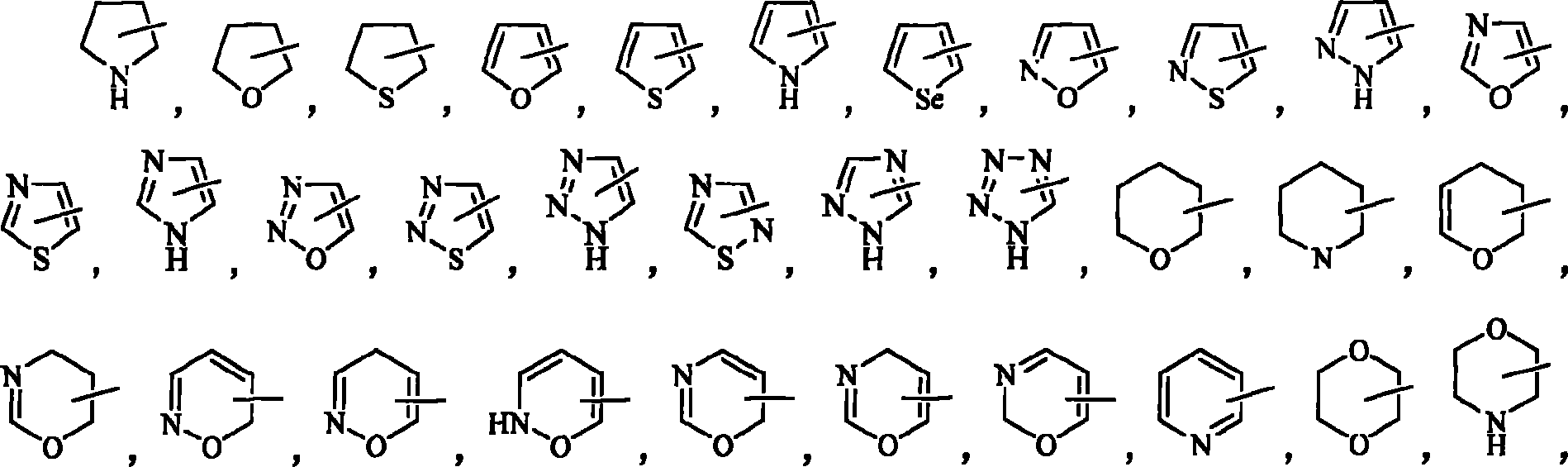
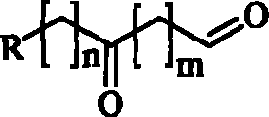Compounds with antimicrobial antiviral activity
A compound and adduct technology, applied in the field of medicine, can solve problems such as increased bacterial resistance and inconvenient clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] Example 1 Preparation of sodium α-hydroxy-[(5-amino-1,2,4-thiadiazol)-3-yl]formyl ethyl sulfonate and its structure confirmation
[0135] (1) Preparation of [(5-amino-1,2,4-thiadiazol)-3-yl]formyl chloride
[0136] Under cooling in an ice bath, mix 29g (0.20mol) of [(5-amino-1,2,4-thiadiazol)-3-yl] formic acid, 100ml of anhydrous acetone, 0.5ml of anhydrous pyridine, and 38g (0.2 mol), stirred at 0°C for 1h, then heated to 15-20°C, stirred for 6 hours, poured into 100ml of ice water, stirred evenly, extracted twice with 50ml of dichloromethane, combined organic layers, dried over anhydrous sodium sulfate , concentrated under reduced pressure at 40°C to 20ml to obtain [(5-amino-1,2,4-thiadiazol)-3-yl]formyl chloride solution, which was sealed for future use.
[0137] (2) Preparation of [(5-amino-1,2,4-thiadiazol)-3-yl]formylacetaldehyde
[0138] Measure 40ml of acetaldehyde in the reaction flask, and slowly add the [(5-amino-1,2,4-thiadiazol)-3-yl]formyl chloride sol...
Embodiment 2
[0142] Example 2 Preparation and Structure Confirmation of Sodium α-Hydroxy-[(2-amino-1,3-thiazol)-4-yl]formylethylsulfonate
[0143] (1) Preparation of [(2-amino-1,3-thiazol)-4-yl]formyl chloride
[0144] Under ice-bath cooling, 28.8g (0.20mol) [(2-amino-1,3-thiazol)-4-yl] formic acid was added in the reaction flask, and the operation method was then referred to step (1) in Example 1 to obtain [(2-Amino-1,3-thiazol)-3-yl]formyl chloride solution, sealed for future use.
[0145] (2) Preparation of [(2-amino-1,3-thiazol)-4-yl]formylacetaldehyde
[0146] The operation method refers to step (2) in Example 1 to obtain an aqueous solution of [(2-amino-1,3-thiazol)-4-yl]formylacetaldehyde, and directly proceed to the next reaction.
[0147] (3) Preparation of α-hydroxyl-[(2-amino-1,3-thiazol)-4-yl]sodium formyl ethyl sulfonate
[0148] The operation method refers to step (3) in Example 1 to obtain 41.8 g of sodium α-hydroxy-[(2-amino-1,3-thiazol)-4-yl]formyl ethyl sulfonate wit...
Embodiment 3
[0150] Example 3 Preparation of sodium α-hydroxy-[(2-methyl-5-nitro-1,3-oxadiazol)-1-yl]acetyl ethyl sulfonate and its structure confirmation
[0151] (1) Preparation of [(2-methyl-5-nitro-1,3-oxadiazol)-1-yl]acetyl chloride
[0152] Under cooling in an ice bath, add 37g (0.20mol) [(2-methyl-5-nitro-1,3-oxadiazol)-1-yl]acetic acid into the reaction flask, and then refer to Example 1 for the operation method In step (1), [(2-methyl-5-nitro-1,3-oxadiazol)-1-yl]acetyl chloride solution is obtained, which is sealed for future use.
[0153] (2) Preparation of [(2-methyl-5-nitro-1,3-oxadiazol)-1-yl]acetoacetaldehyde
[0154] The operation method refers to step (2) in Example 1 to obtain an aqueous solution of [(2-methyl-5-nitro-1,3-oxadiazol)-1-yl]acetoacetaldehyde, and directly proceed to the next reaction.
[0155] (3) Preparation of α-hydroxy-[(2-methyl-5-nitro-1,3-oxadiazole)-1-yl] sodium acetyl ethyl sulfonate
[0156]The operation method refers to (c) in Example 2 to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com