Biomarkers for breast cancer
A biomarker, technology for breast cancer, applied in the field of clinical diagnostics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0170] 11.1. Example 1. Discovery of breast cancer biomarkers
[0171] It is generally understood that the embodiments and specific embodiments described herein are for illustrative purposes only, and for those skilled in the art, various modifications or changes according to these embodiments are also considered to have been suggested by the present case and included in the within the spirit and scope of the invention and within the purview of the appended claims. All publications, patents, and patent applications cited herein are hereby incorporated by reference for all purposes.
Embodiment 2
[0172] 11.2. Example 2. Material Level Methods
[0173] 11.2.1. Multi-center study design
[0174] To minimize potential bias in patient selection and fluid collection procedures at each institution, and to maximize the applicability of this finding, we selected three institutions: University of Texas M.D. Anderson Cancer Center; Sidney Kimmel Comprehensive Cancer Center in Johns Hopkins Hospital; And Department of Surgery, Feinberg School of Medicine, Northwestern University enlisted two liquid samples for nipple aspiration and milk duct lavage. Sample collection at each site was approved by the respective Institutional Review Board, and current proteosome studies were approved by the Institutional Review Board of Johns Hopkins University.
[0175] 11.2.2. Patient - Nipple Aspiration Fluid (NAF)
[0176] NAF samples were obtained from the University of Texas M.D. Anderson Cancer Center. Patients eligible for bilateral nipple aspiration were those with biopsy-proven stage I...
Embodiment 3
[0198] 11.3. Example 3. Results
[0199] 11.3.1. Proteome profile of breast fluid
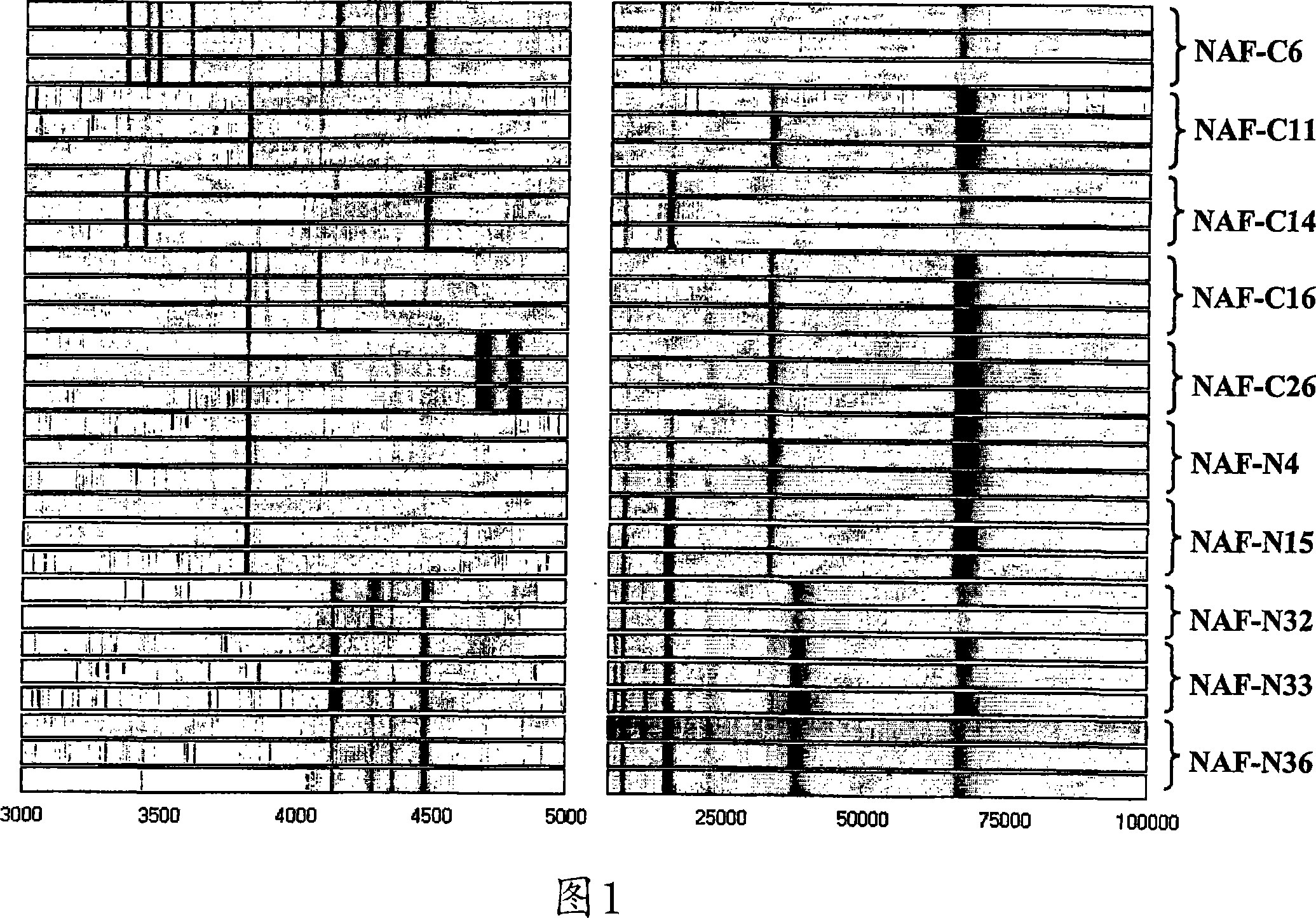
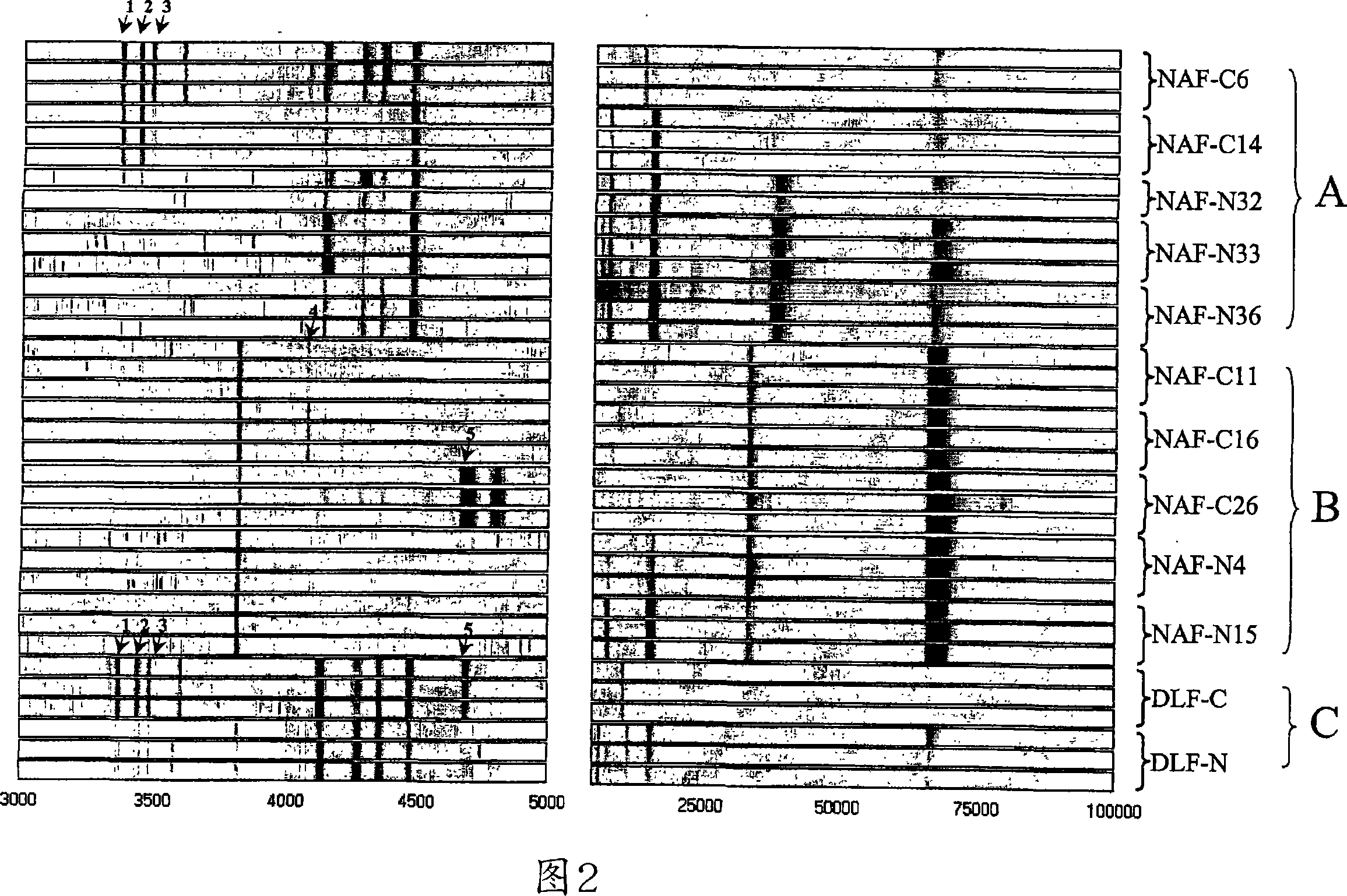
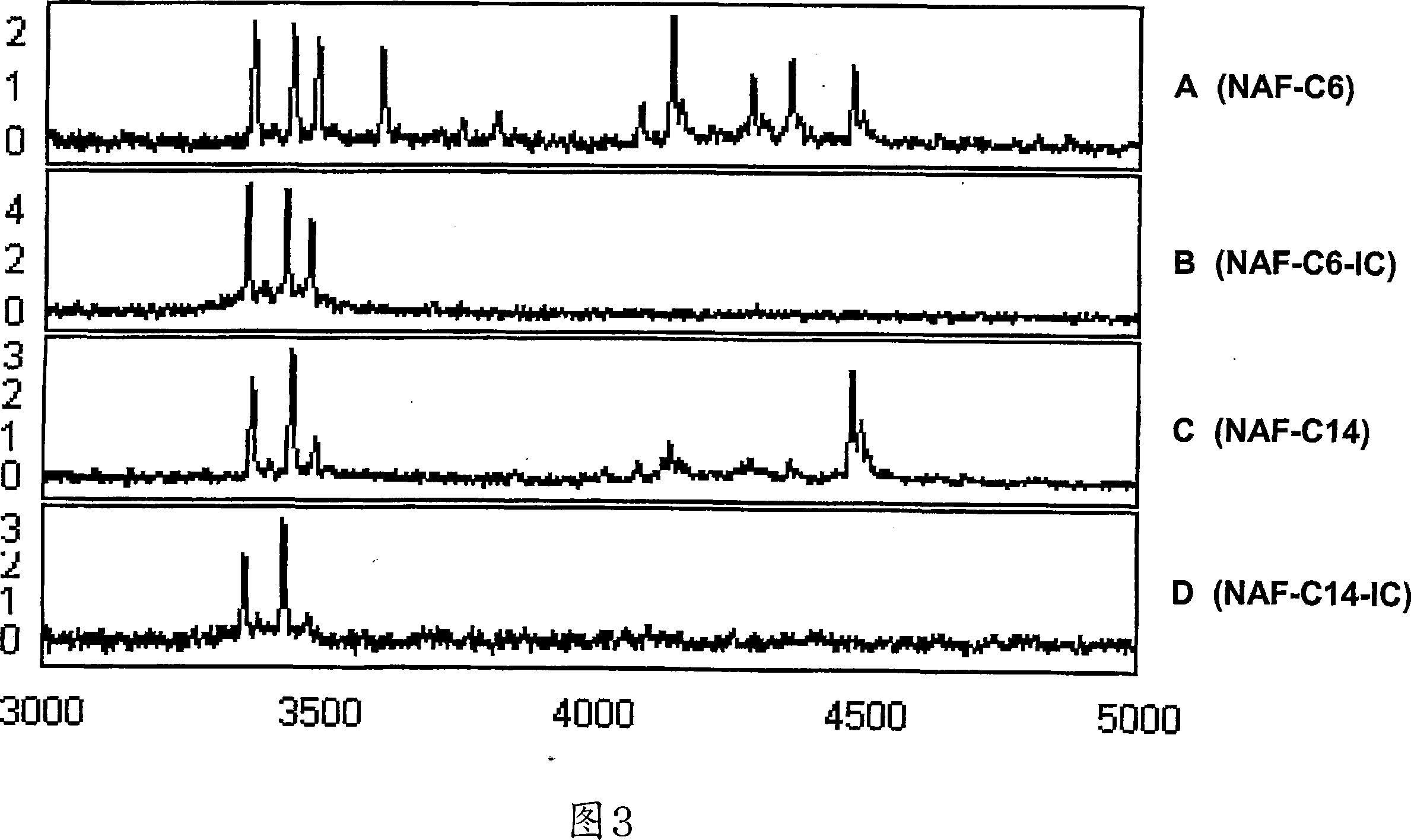
[0200] Reproducible protein profiles were obtained using our optimized microarray procedure and breast fluid samples containing 1 μg of whole protein. Figure 1 shows nipple suction fluid from 5 cancerous breasts (C6, C11, C14, C16, C26) of patients with primary invasive cancer and 5 normal control breasts (N4, N15, N32, N33, N36) Pseudo-gel map of the protein map. The general protein expression profile varies from subject to subject, whereas mass spectra from triplicate analyzes of the same specimen are highly reproducible. M / Z (mass / charge) ion signals less than 3000 are mainly matrix material noise, and a maximum M / Z is detected at 135,000. We have manually selected 73 protein peaks (signal / noise >5, M / Z from 3K to 135K) for subsequent biomarker evaluation.
[0201] 11.3.2. Using training data to select biomarkers
[0202] Use 10 NAF samples as training samples. First, we performed an u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com