Polyoxyalkylene chain-containing lipid derivative and lipid film structure containing such derivative
A technology of lipid derivatives and polyoxyalkylene chains, which can be used in liposome delivery, oil/fat/wax non-active ingredients, drug combinations, etc., and can solve the problem of drug activity reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
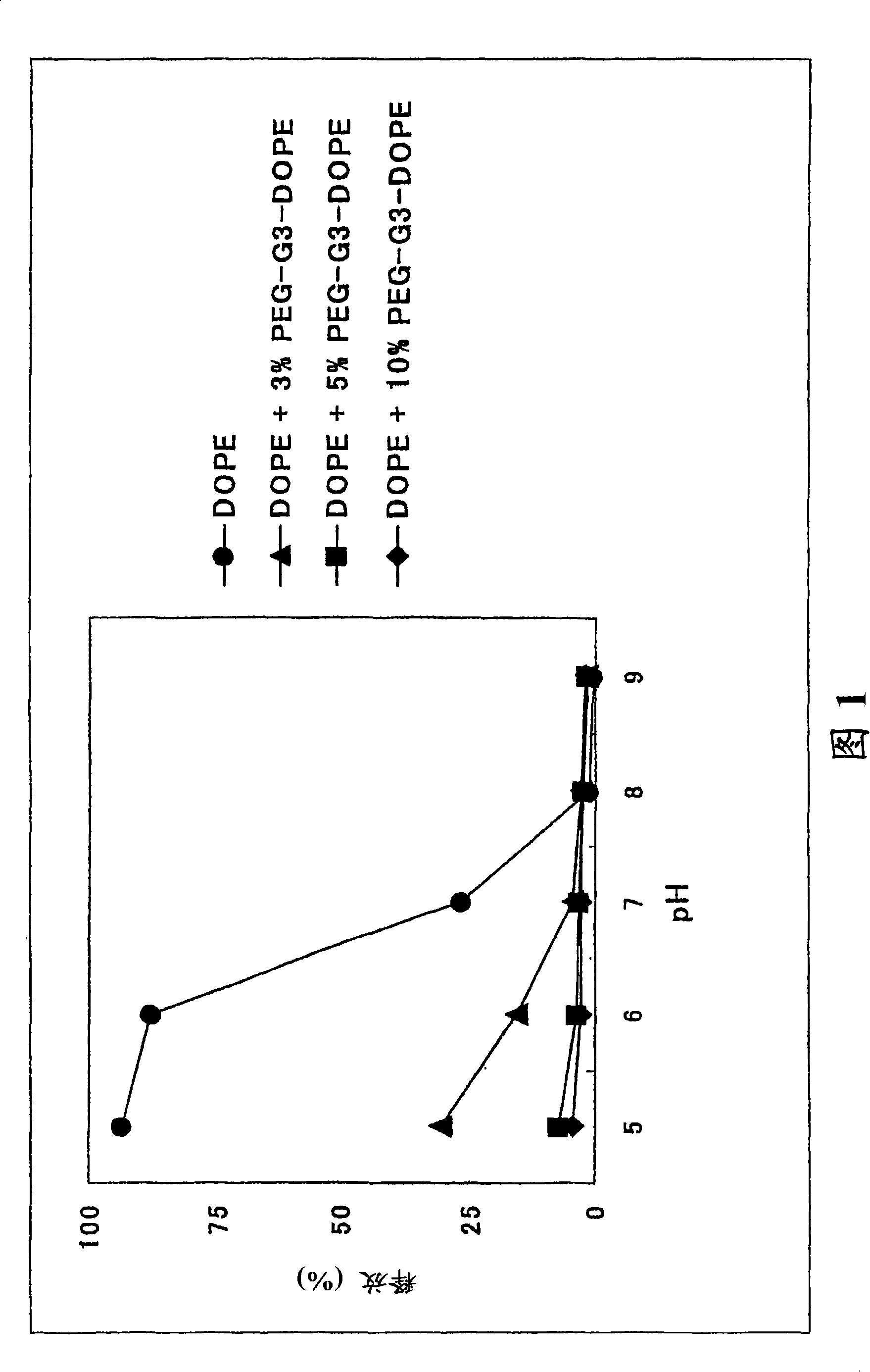
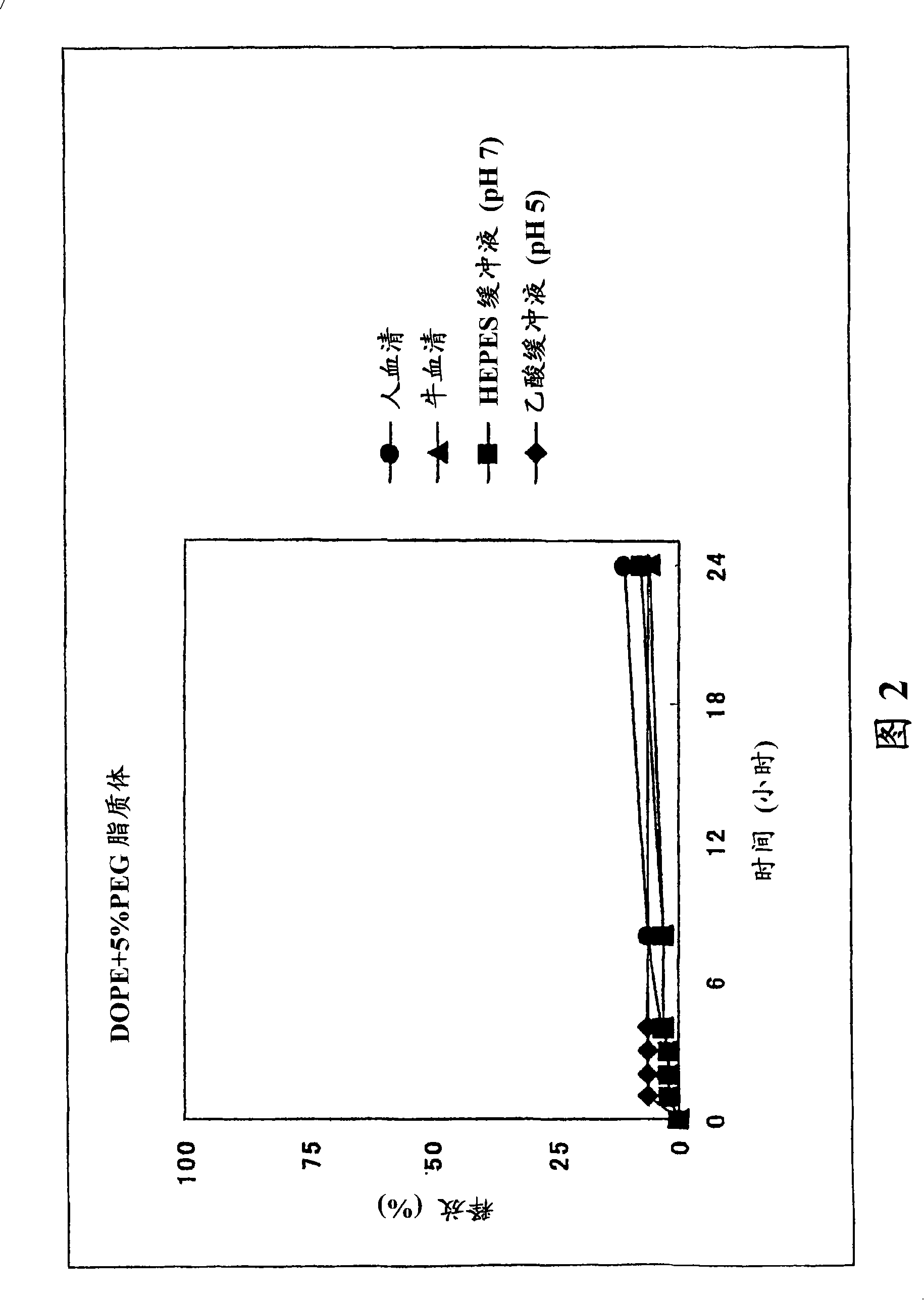
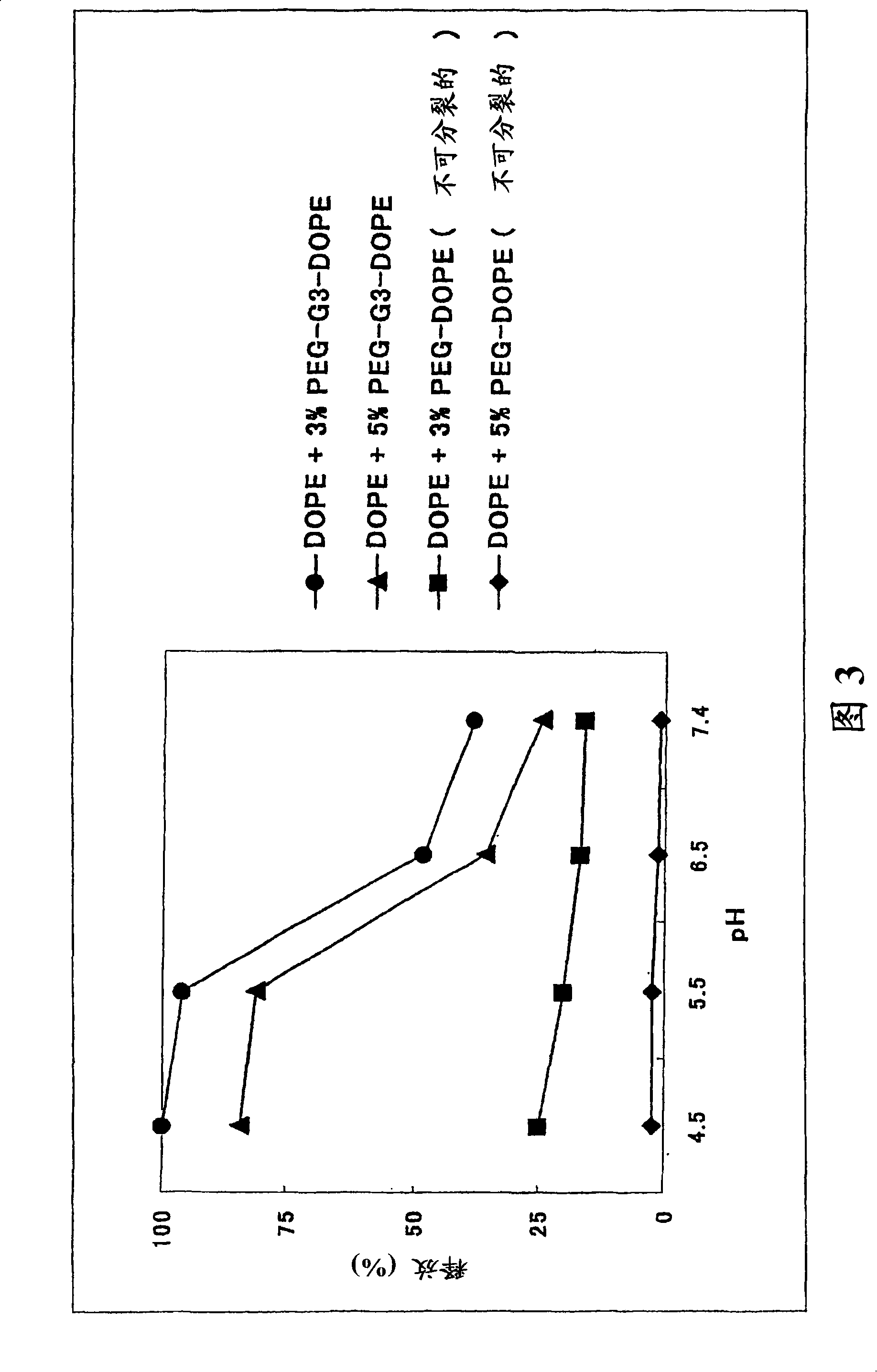
Image
Examples
Embodiment 1
[0152] Synthesis of Methylpolyoxyethylene Carbamoyl-Glycyl-Glycyl-Glycyl-Dioleoyl Phosphatidylethanolamine (DOPE-GGG-PEG2000)
[0153] (1) Synthesis of methyl polyoxyethylene carbamoyl-glycyl-glycyl-glycine 5g (2.5mmol) methoxy polyethylene glycol p-nitrophenyl carbonate (trade name: SUNBRIGHT MENP- 20H, weight average molecular weight 2,000, manufactured by NOF Co., Ltd.) was dissolved in 15 mL of acetonitrile. To this solution, an aqueous solution of 946 mg of glycyl-glycyl-glycine dissolved in 10 mL of water was added, stirred, and 0.5 g of triethylamine was added, followed by stirring at room temperature for 3 hours. After the reaction, the insoluble matter was removed by filtration, and then the solvent was removed under reduced pressure using an evaporator. Next, 50 mL of ethyl acetate was added to dissolve, and after adding sodium sulfate and stirring, it was filtered and dehydrated. Next, 100 mL of hexane was added to the filtrate, cooled to below 0° C., and filtered...
Embodiment 2
[0159] Methylpolyoxyethylenecarbamoyl-glycyl-lysine (methylpolyoxyethylenecarbamoyl)-glycyl-glycyl-glycyl-distearoylphosphatidylethanolamine (DSPE-GGG Synthesis of -K(PEG2000)-G-PEG2000)
[0160] (1) Synthesis of methyl polyoxyethylene carbamoyl-glycyl-lysine (methyl polyoxyethylene carbamoyl)-glycyl-glycyl-glycyl
[0161] Dissolve 10 g (5 mmol) of methoxypolyethylene glycol-p-nitrophenyl carbonate (SUNBRIGHTMENP-20H, weight average molecular weight 2,000, manufactured by NOF Corporation) in 30 mL of acetonitrile, and add 1.6 An aqueous solution of g glycyl-lysine-glycyl-glycyl-glycine (sequence number 11) dissolved in 18 mL of water was stirred, and 1 g of triethylamine was added, and stirred at room temperature for 3 hours. After the reaction, the insoluble matter was removed by filtration, and then the solvent was removed under reduced pressure using an evaporator. Next, 50 mL of ethyl acetate was added and dissolved, and sodium sulfate was added and stirred, followed by ...
Embodiment 3
[0167] Methylpolyoxyethylene carbamoyl-cysteine (methylpolyoxyethylene maleimide)-glycyl-glycyl-distearoylphosphatidylethanolamine (DSPE-GGC(-PEG2000)- PEG5000) synthesis
[0168] (1) Synthesis of methyl polyoxyethylene carbamoyl-cysteine
[0169]Add 150 ml (0.1 M, pH 9.5) of boric acid buffer solution to 2.5 g of L-cystine, and stir until completely dissolved. Dissolve 50 g (10 mmol) of methoxypolyethylene glycol-p-nitrophenyl carbonate (trade name: SUNBRIGHT MENP-50H, average molecular weight 5000, produced by NOF Corporation) in 50 mL of water, and add to the previous L - In cystine, keep the pH at 9.5 and react at room temperature for 2 hours. After the reaction, the pH was adjusted to 5.5 with dilute hydrochloric acid, and cooled to 2°C. Next, insoluble matter was removed by filtration, and the filtrate was dialyzed 5 times against 2 L of ion-exchanged water using a dialysis tube. After the dialysis, the pH was adjusted to 7.5, the solution was cooled to 2° C., and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com