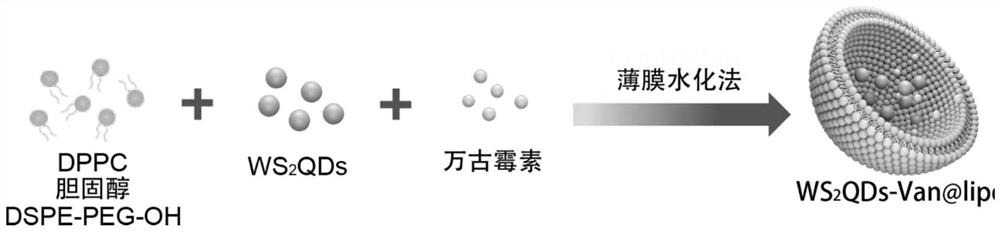
Preparation method and application of liposome composite material for light-controlled release of tungsten sulfide quantum dots and vancomycin
A technology of vancomycin and light-controlled release, which is applied in the application of antibacterial reagents, preparation of liposome composite materials, and the field of antibacterial reagents, and can solve problems such as limiting the application of CDT
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A light-controlled release WS 2 The preparation method of the liposome composite material of QDs and vancomycin, the specific preparation method comprises the following steps:
[0046] Step 1: Dissolving dipalmitoylphosphatidylcholine, cholesterol and distearoylphosphatidylethanolamine-polyethylene glycol-hydroxyl in chloroform at a molar ratio of 20:10:1, and ultrasonically assisted dissolution, Subsequently, by vacuum drying for 30 minutes, a lipid film was obtained on the inner wall of the reaction vessel;
[0047] Step 2: Dissolve tungsten sulfide quantum dots and vancomycin in PBS buffer, then add the PBS buffer containing tungsten sulfide quantum dots and vancomycin into the reaction vessel containing lipid film, and vortex at 45°C Rotate and shake for 1 hour to obtain a hydration solution;
[0048] Step 3: The obtained hydration solution is ultrasonically treated, the ultrasonic power is 60W, and the ultrasonic time is 5 minutes. Finally, it is centrifuged and ...
Embodiment 2
[0050] The invention also provides a light-controlled release WS 2 The application of the liposome composite material of QDs and vancomycin, the method prepared in the embodiment one prepares a kind of liposome composite material of light-controlled release tungsten sulfide quantum dot and vancomycin, namely WS 2 QDs - Van @lipo.
[0051] The application of the antibacterial composite material, the liposome composite material of light-controlled release of tungsten sulfide quantum dots and vancomycin, under near-infrared light and low dose H 2 o 2 Under certain conditions, it has broad-spectrum antibacterial activity against bacteria including but not limited to Escherichia coli and vancomycin-resistant Staphylococcus aureus.
[0052] The application of the antibacterial composite material, the liposome composite material of light-controlled release of tungsten sulfide quantum dots and vancomycin, under near-infrared light and low dose H 2 o 2 Under certain conditions, it ...
Embodiment 3
[0054] Refer to the attached Figure 1-4 Shown:
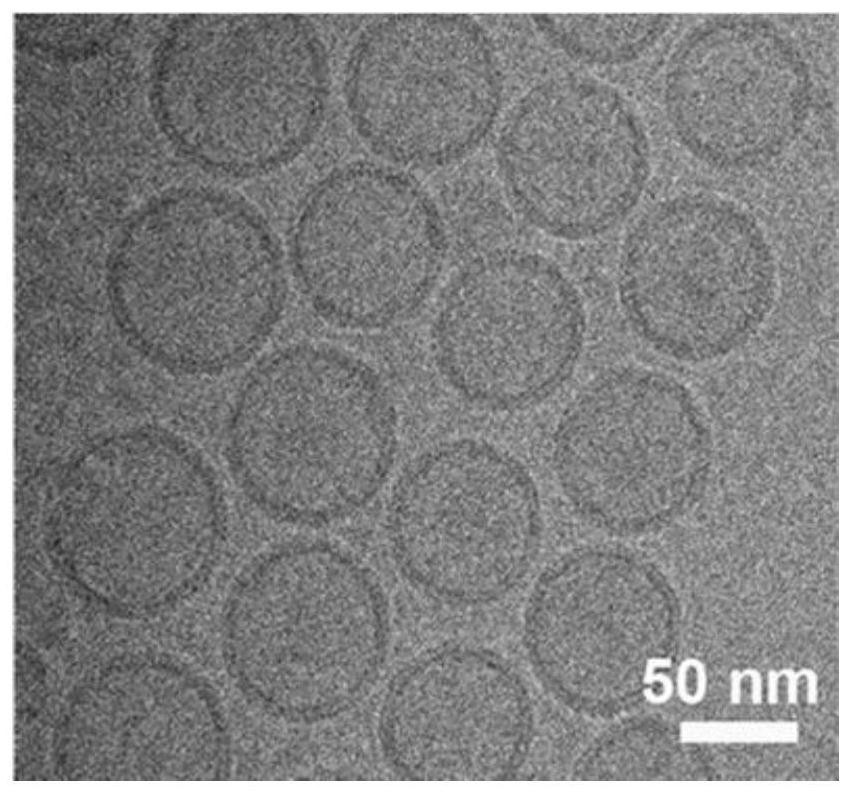
[0055] In the release of WS on light control 2 During the preparation of liposome composites of QDs and vancomycin, WS 2 QDs-Van@lipo; WS characterized using cryo-electron microscopy 2 The morphology and size of QDs-Van@lipo, found that the nanoparticles are uniform spherical, with an average size of less than 100nm;
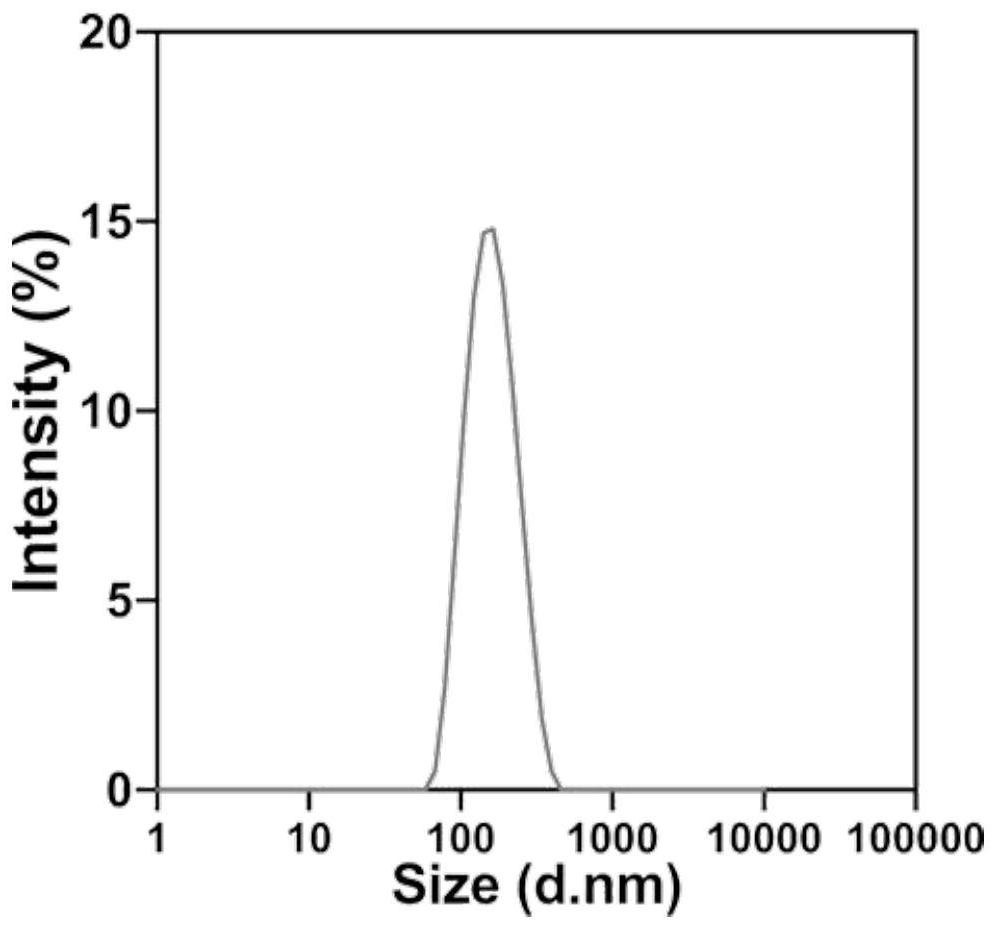
[0056] Subsequent to WS 2 Hydrated particle size (DLS) measurement by QDs-Van@lipo, WS 2 The average particle size measured by QDs-Van@lipo is about 146.37±0.67nm;
[0057] to WS 2 Zeta Potential Measurements by QDs-Van@lipo, WS 2 The measured surface zeta potentials of QDs-Van@lipo are -35.4 mV, respectively, and these results confirm the designed WS 2 Successful synthesis of QDs-Van@lipo nanomaterials.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com