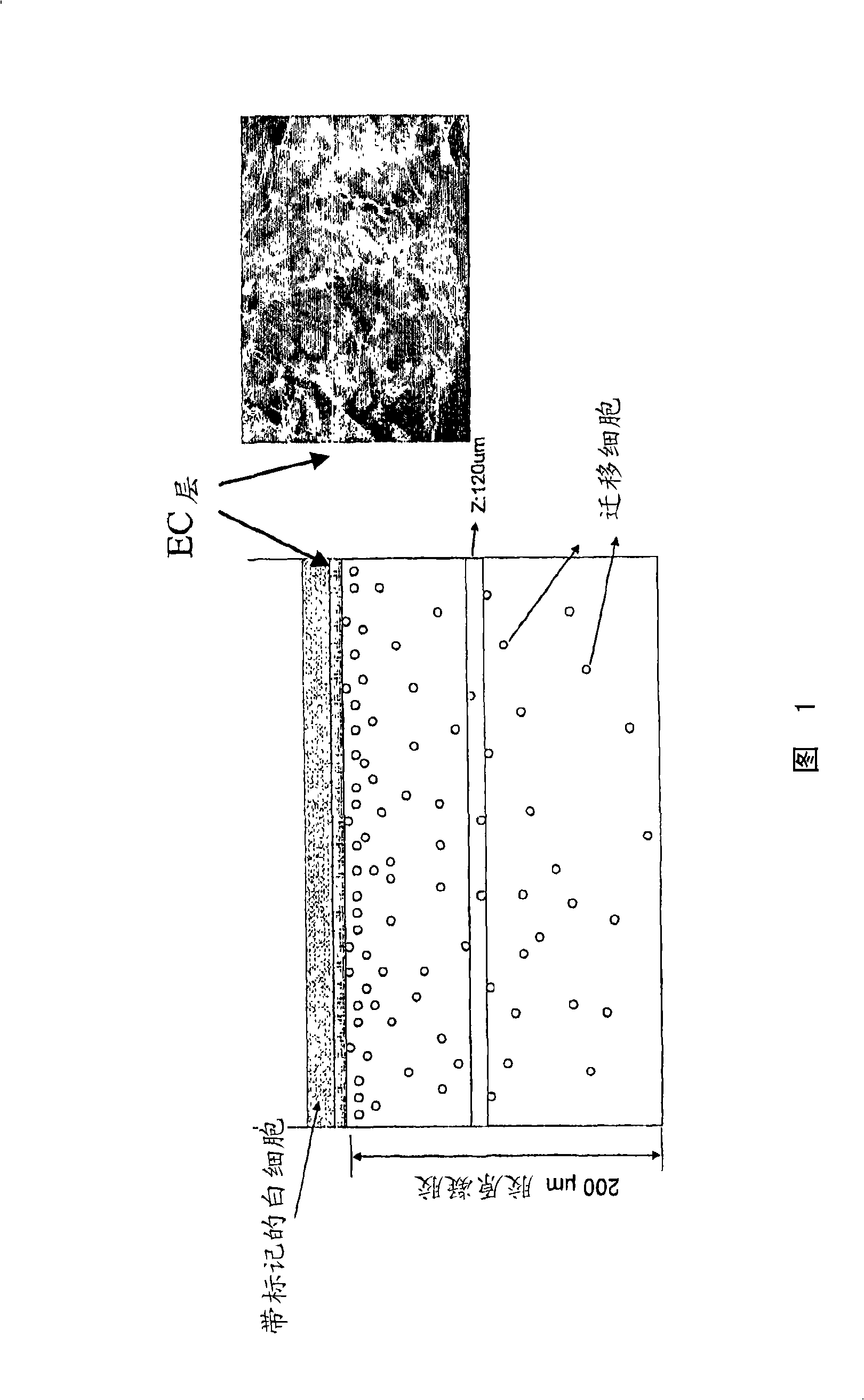
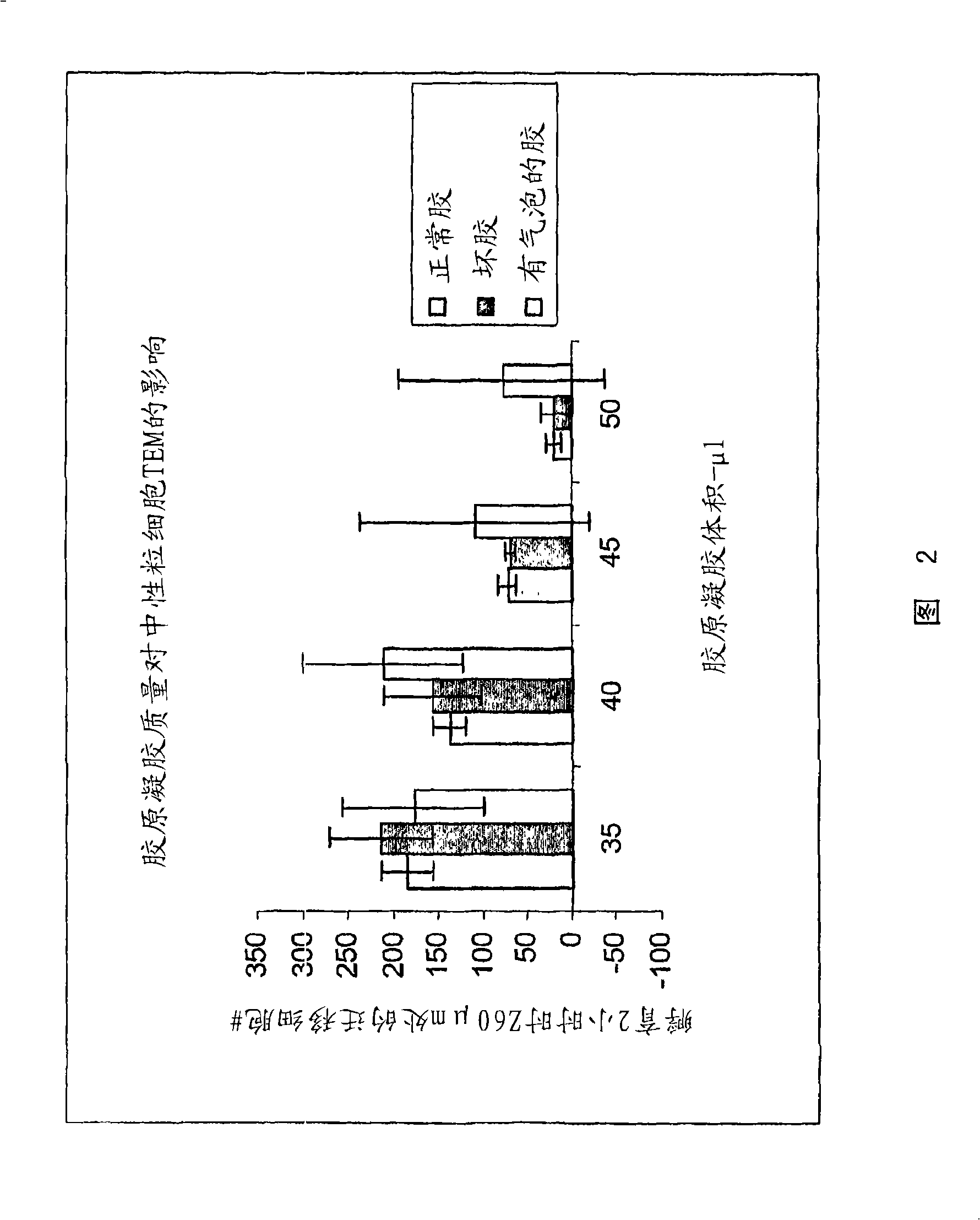
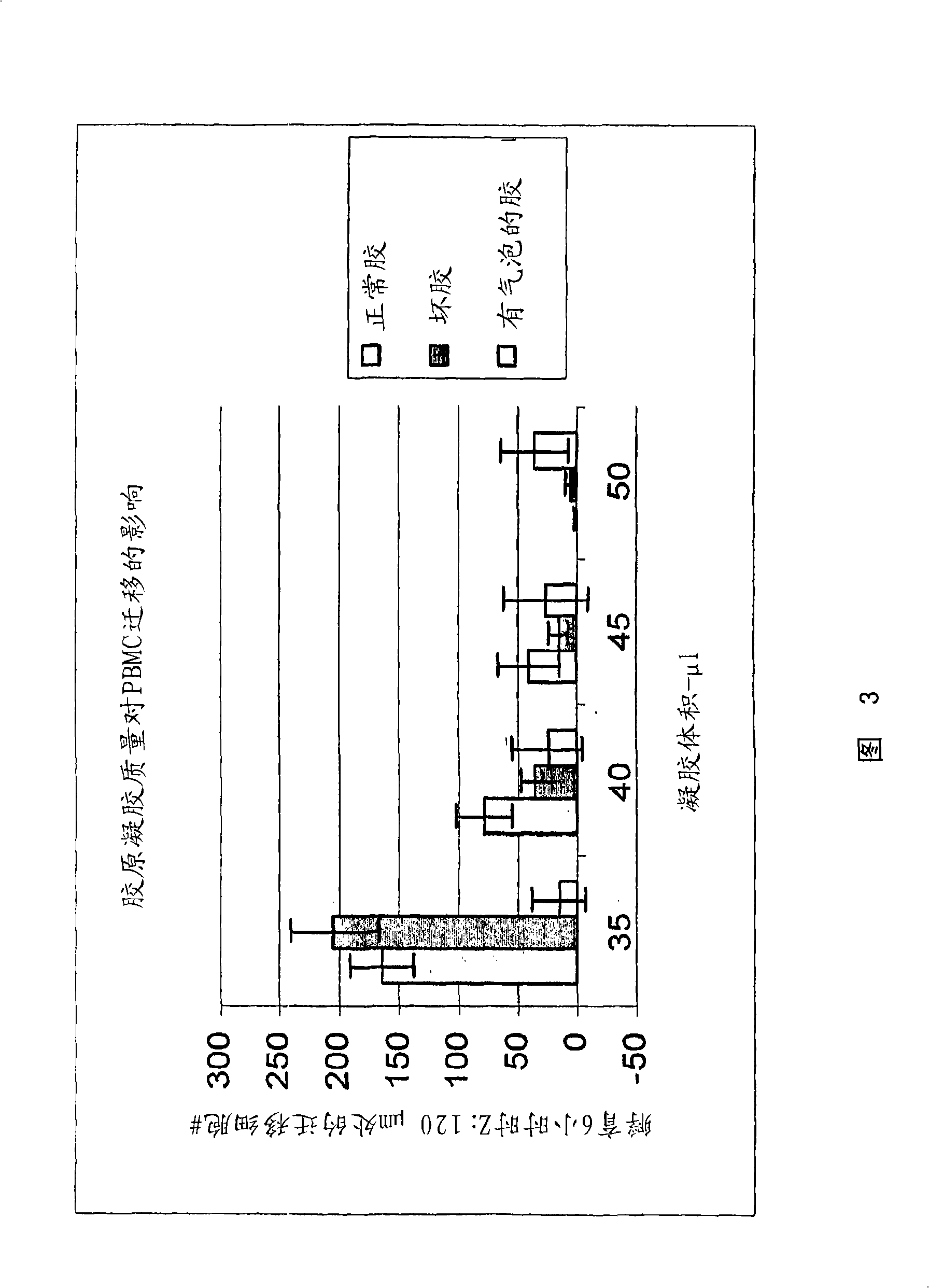
Cell migration assay
A cell migration, cell technology, applied in the field of preparation and use of these compositions, compositions for transendothelial migration assays, can solve problems such as being unsuitable for high-throughput screening, complex preparation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] Preparation of the composition
[0035] We provide detailed materials and methods used to prepare the assay system in the Examples section. Briefly, collagen is deposited in the container and solidifies to form a solid layer. Or alternatively, synthetic matrix gels that support 3D endothelial growth and cell migration can be used. Optionally, a gelatin solution is added to the collagen gel before the collagen layer solidifies. The first cell type (endothelial cells) is then placed on the solid layer and cultured to form a confluent layer of cells in contact with the solid layer. Migrated cells are then prepared and seeded on top of a confluent layer of the first cell type. Typically, the first cell type is endothelial cells, such as HUVECs. Other primary endothelial cells such as HCAEC (coronary artery endothelial cells), HMVEC (pulmonary microvascular endothelial cells) or endothelial cell lines such as SK-HEP-1 (ATCC HTB-52) can also be used. Although any migra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com