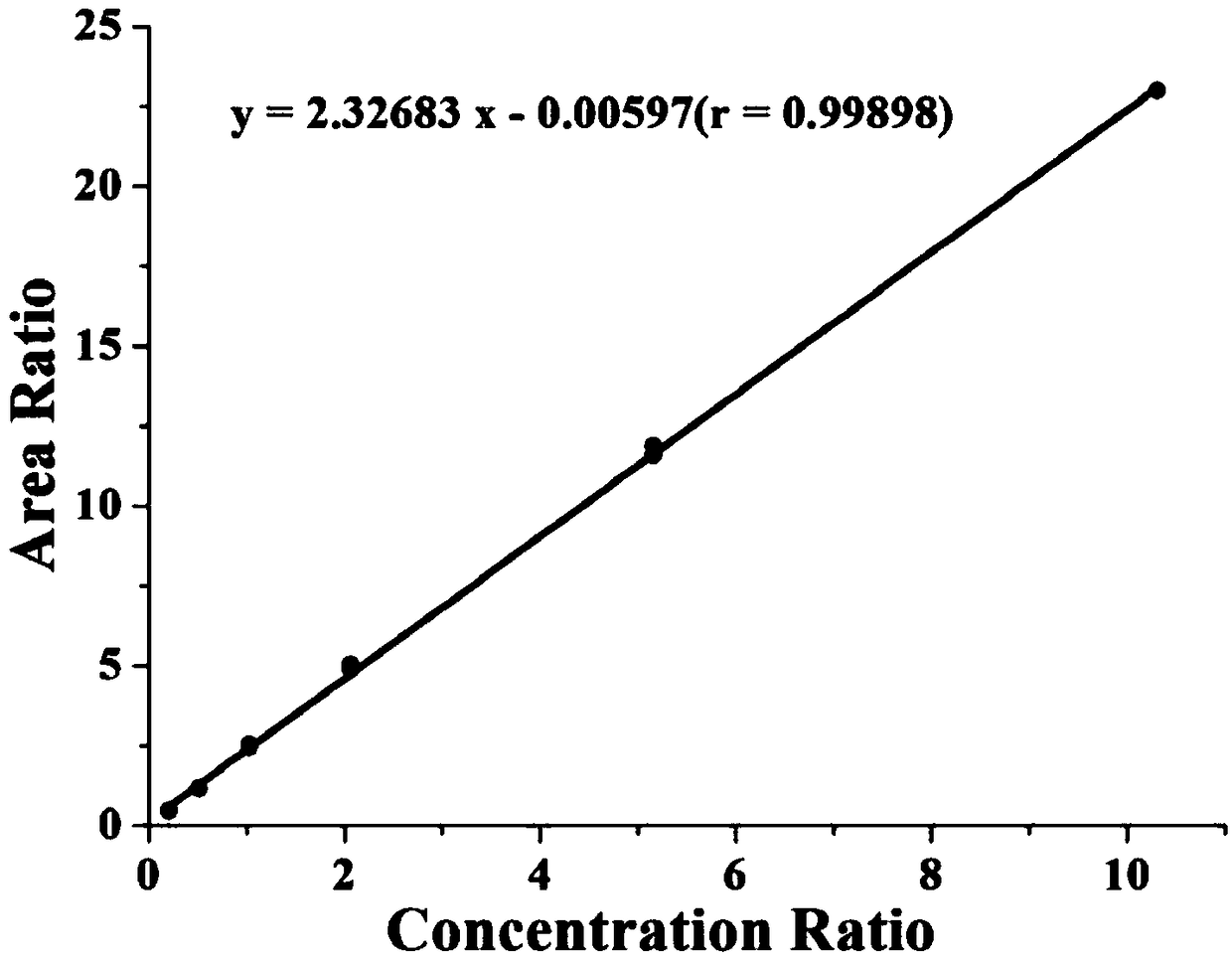
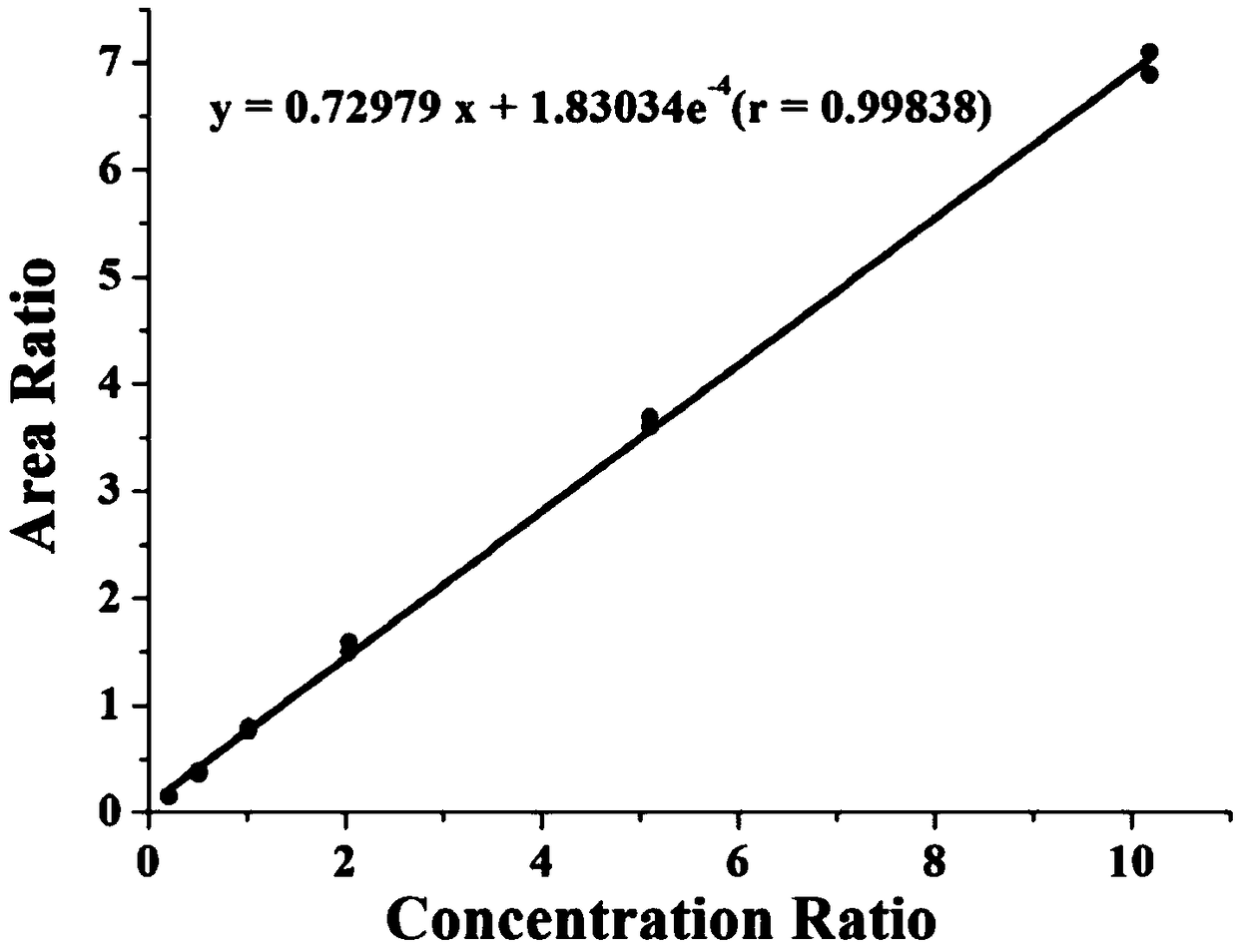
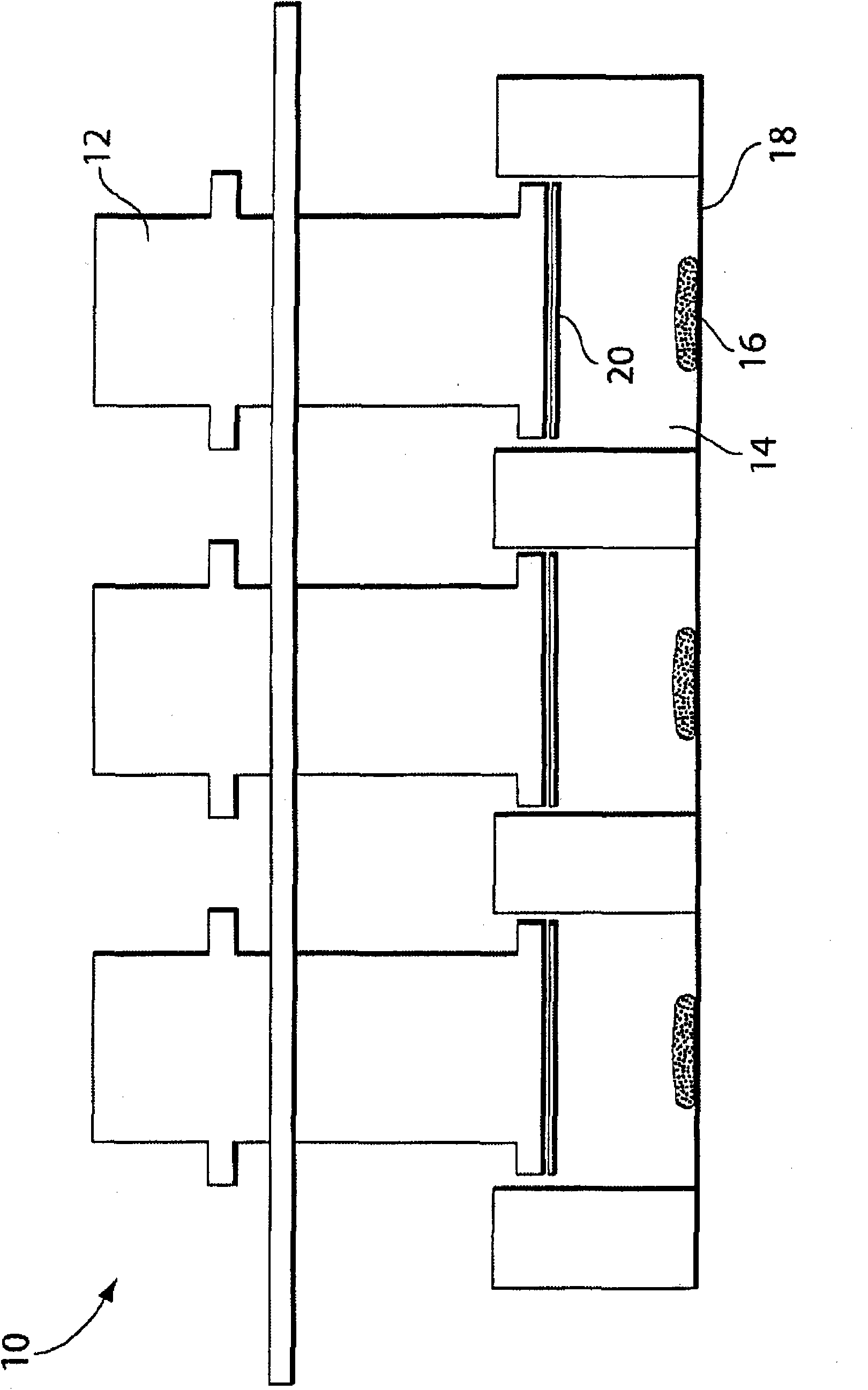
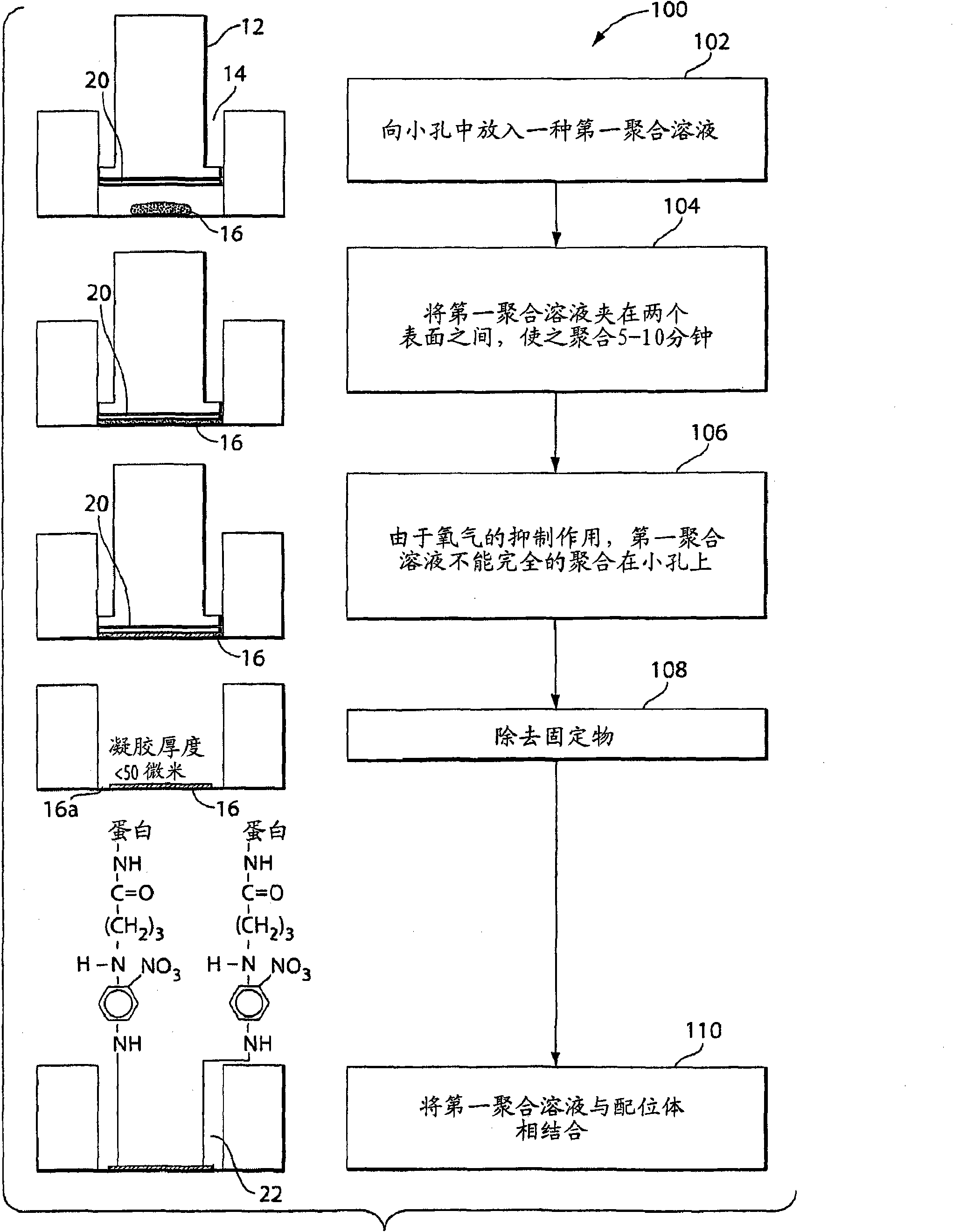
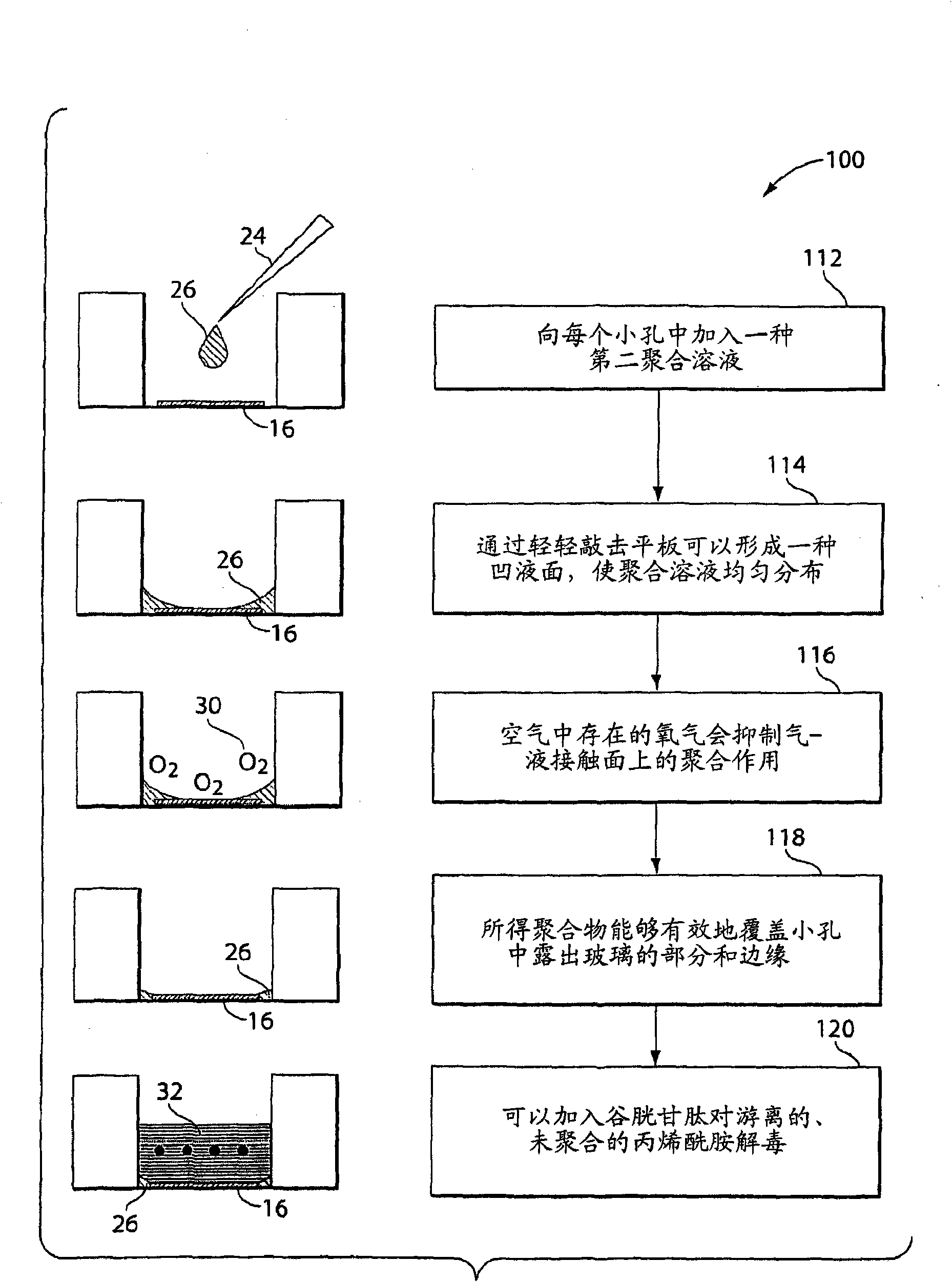
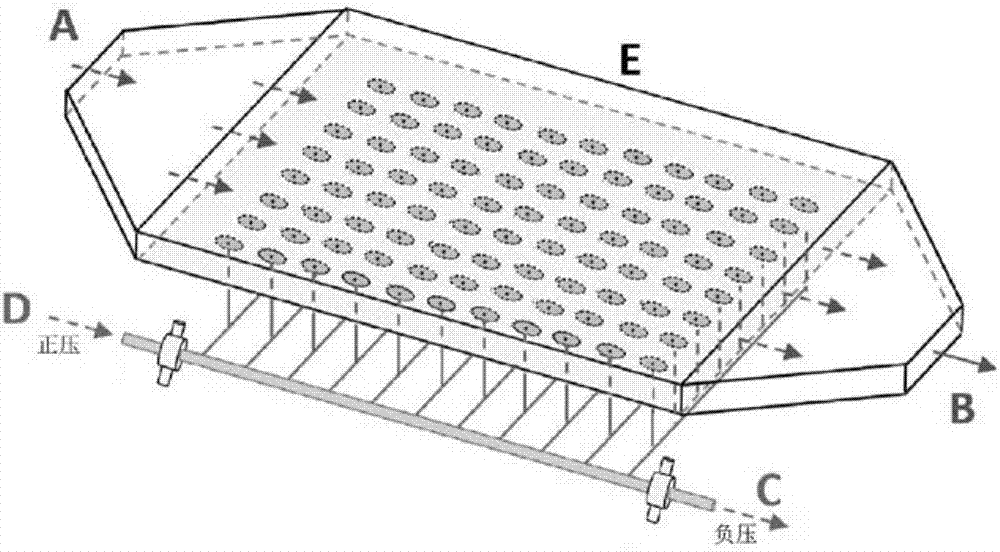
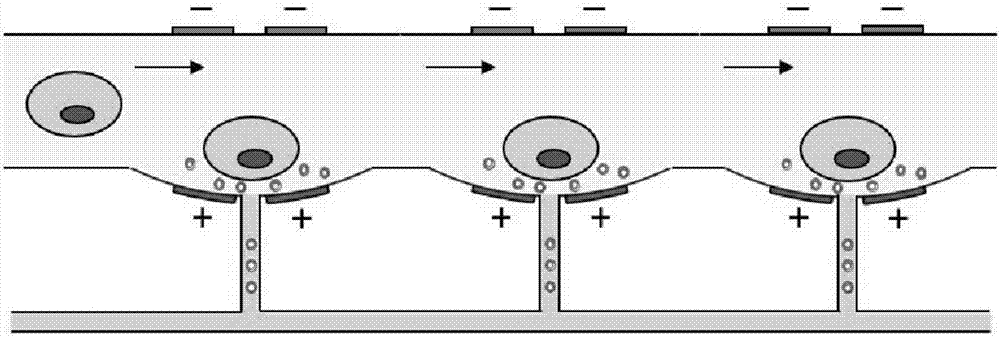
Patents
Literature
148 results about "96 well plate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Device, system and method for extracting and preparing brain tissue
InactiveUS20050256425A1Easy to useQuick extractionSurgical needlesVaccination/ovulation diagnosticsCuffBrain tissue
A syringe type tissue extraction device is disclosed. The device comprises a sharp, cylindrical metal cuff at one end of a transparent tube which is pushed into brain tissue. Once inserted a plunger is pulled back and by observation through the transparent tube a determined amount of tissue is extracted. The device is then used to place the sample in a single homogenization tube or to punch through a seal above a well of a 96 well plate and the tissue in the tube is inserted into the well. Each well (or the single tube) of the plate holds beads and once tissue is inserted in all the wells the plate is shaken to homogenize the tissue in each well in order to prepare the sample for assaying.
Owner:INPRO BIOTECH
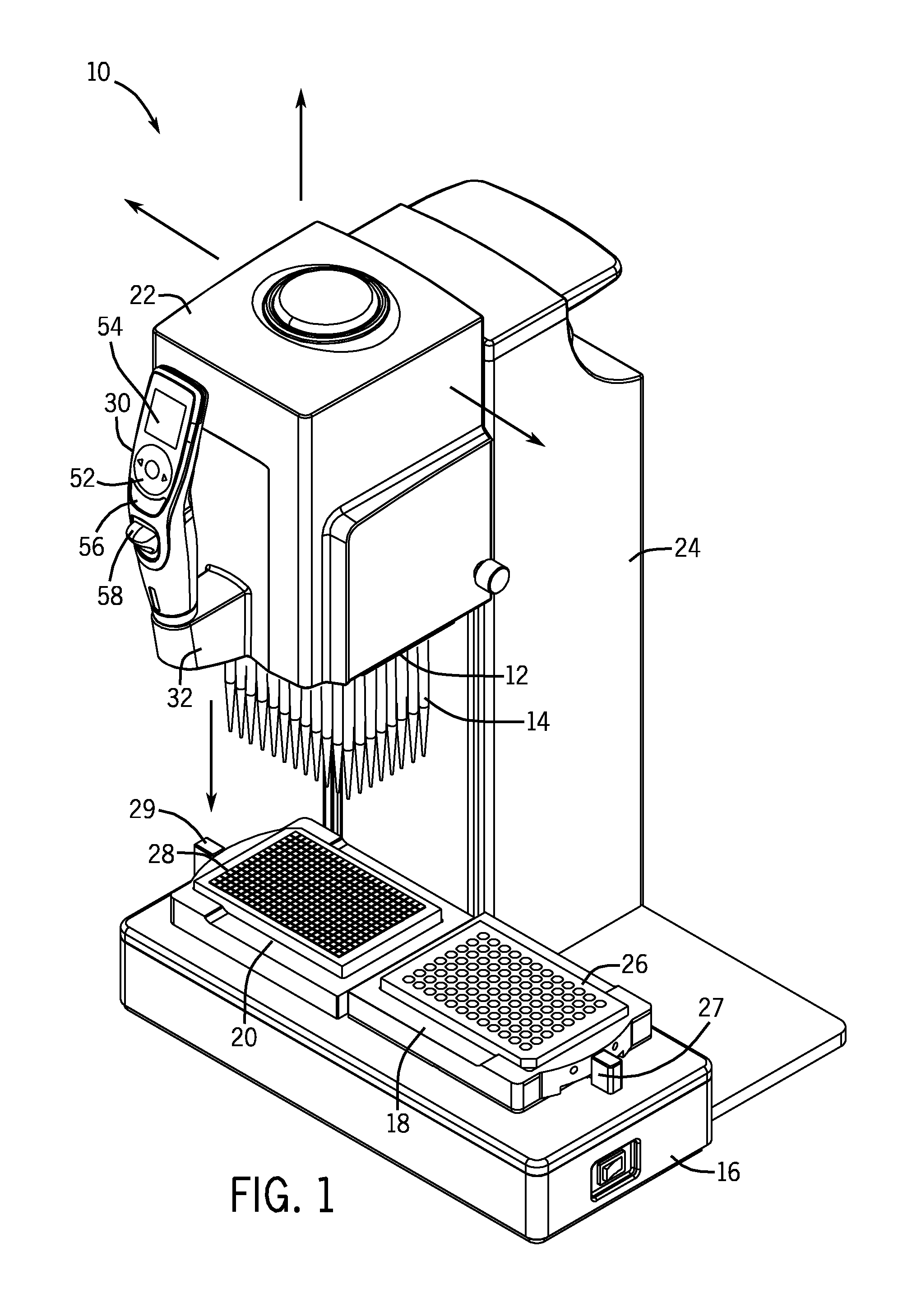
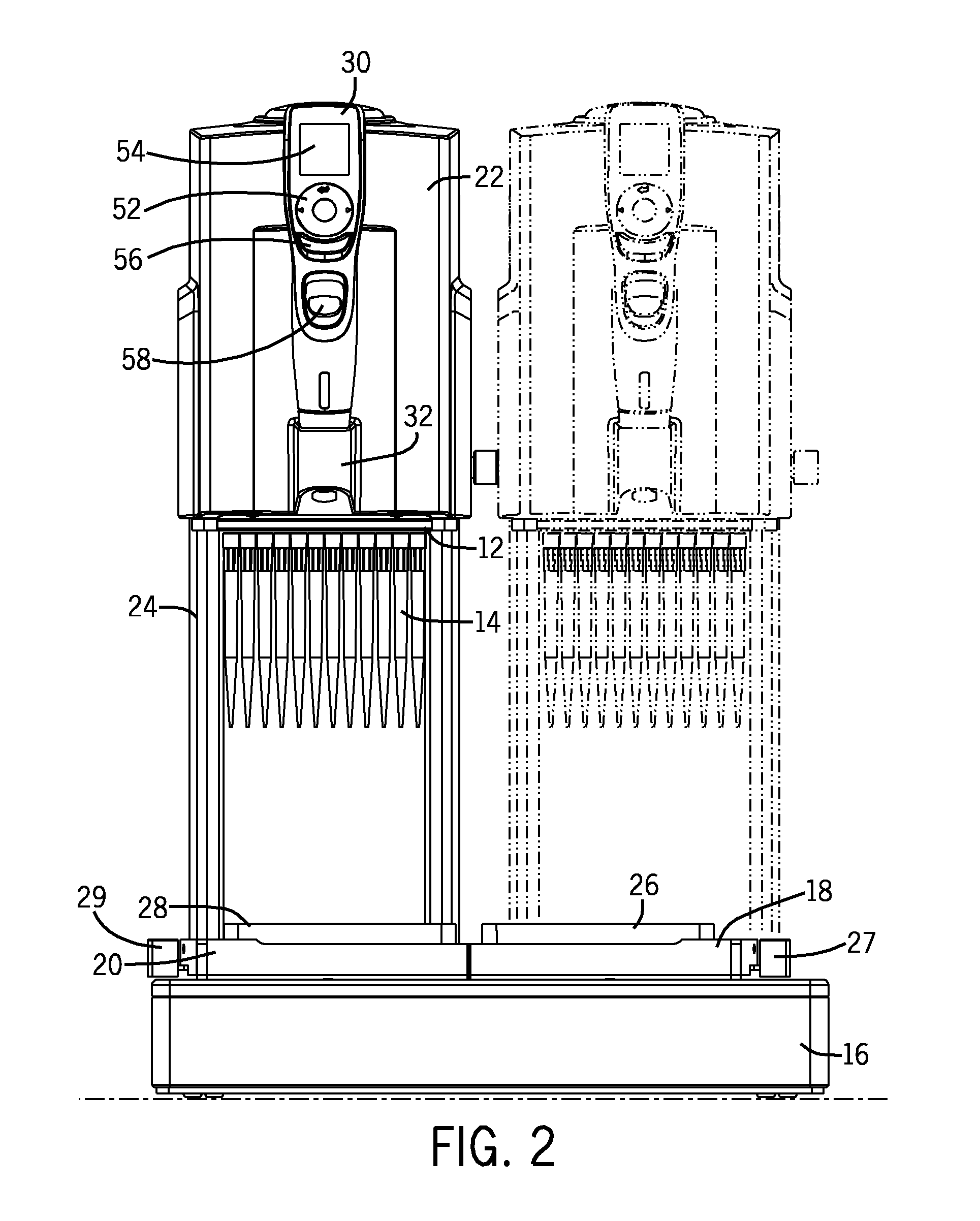
Pipette Tip Positioning For Manually-Directed, Multi-Channel Electronic Pipettor
ActiveUS20110296931A1Alignment accuracyPrecise alignmentBurettes/pipettesMaterial analysisTip positionPipette
A manually directed, multi-channel electronic pipettor includes a software biasing mode to assure proper alignment over wells in the 96-well plate and the 384-well plate. The system also includes manual repositioning levers for nesting receptacles which are customized for 96 well-plates and 384 well-plates respectively.
Owner:INTEGRA BIOSCI CORP
Method for diagnosing colon cancer
The present invention relates to a method for diagnosing colon cancer by detecting a colon cancer specific antigen, defensin α6 from the blood of patient and a diagnostic kit for colon cancer comprising anti-defensin α6 antibody. The diagnostic kit for colon cancer of present invention comprises: a solid support such as 96-well plate for ELISA, nitrocellulose membrane, polyvinylidene fluoride membrane, microplate, glass substrate, polystyrene substrate, silicone substrate or metal plate, on which anti-defensin α6 antibody is immobilized; and, a means for detecting colon cancer specific antigen such as a primary antibody which specifically binds with an antigen conjugated with an antibody on a solid substrate and a secondary antibody-signal complex which specifically binds with the primary antibody. The diagnostic kit of the invention can diagnose colon cancer with the minute amount of patients' blood, which makes possible the easy and simple diagnosis of colon cancer.
Owner:RNL BIO
Indirect ELISA kit for detecting African swine fever virus antibody and application thereof
InactiveCN102236017AImmunoglobulinsMaterial analysisAfrican swine fever virus AntibodyPositive control
The invention discloses an indirect ELISA kit for detecting an African swine fever virus antibody and an application thereof, and belongs to the technical field of biology. The kit adopts prokaryotic expression recombinant P30 protein as a coating antigen, and detects the antibody of African swine fever virus in porcine serum based on the indirect ELISA principle. The coating antigen in a 96-well plate of the kit is prokaryotic expression recombinant P30 protein which has good antigenicity. The enzyme-linked immunoassay kit provided by the invention comprises a 96-well plate coated with P30 protein, a positive control, a negative control, a horseradish peroxidase-labeled rabbit anti-porcine IgG polyclonal antibody, a concentrated washing liquid, a serum diluent, a TMB substrate, and a terminating liquid. The kit of the invention is applicable to the screening of large quantities of samples, and main reagents in the kit are provided in a form of operating fluid which is convenient for use.
Owner:陈文刚
High performance liquid chromatography tandem mass spectrometry detection method of 25-hydroxy vitamin D in serum
InactiveCN108645942AStrong specificityIncrease polarityComponent separationDerivatizationTandem mass spectrometry
The invention discloses a high performance liquid chromatography tandem mass spectrometry detection method of 25-hydroxy vitamin D in serum. The method comprises the following steps that (1) a sampleis pre-treated, specifically, an internal standard working solution and a sodium hydroxide solution are sequentially added into a serum sample and mixed to be uniform; (2) solid-liquid extraction treatment is conducted, specifically, the sample treated in the step (1) is loaded to an SLE 96 pore plate, and eluted with normal hexane, and then the eluant is blown to be dried by using nitrogen; (3) aderivatization reaction is conducted, a derivatization reagent 4-phenyl-1,2,4-triazoline-3,5-diketone (PTAD) solution is added to redissolve, a thermostatic reaction is conducted, then ethyl alcoholis added to terminate the reaction, and the product is blown to be dried by using nitrogen; and (4) redissolution is conducted, and analytical detection is conducted by adopting the high performance liquid chromatography tandem mass spectrometry based on a multiplexing system (MPX). The high performance liquid chromatography tandem mass spectrometry detection method is high in detection sensitivity, high in specificity and low in cost, meanwhile, the pre-treatment time and analysis time of the sample are effectively shortened, and the detection efficiency of the sample is improved.
Owner:杭州凯莱谱精准医疗检测技术有限公司
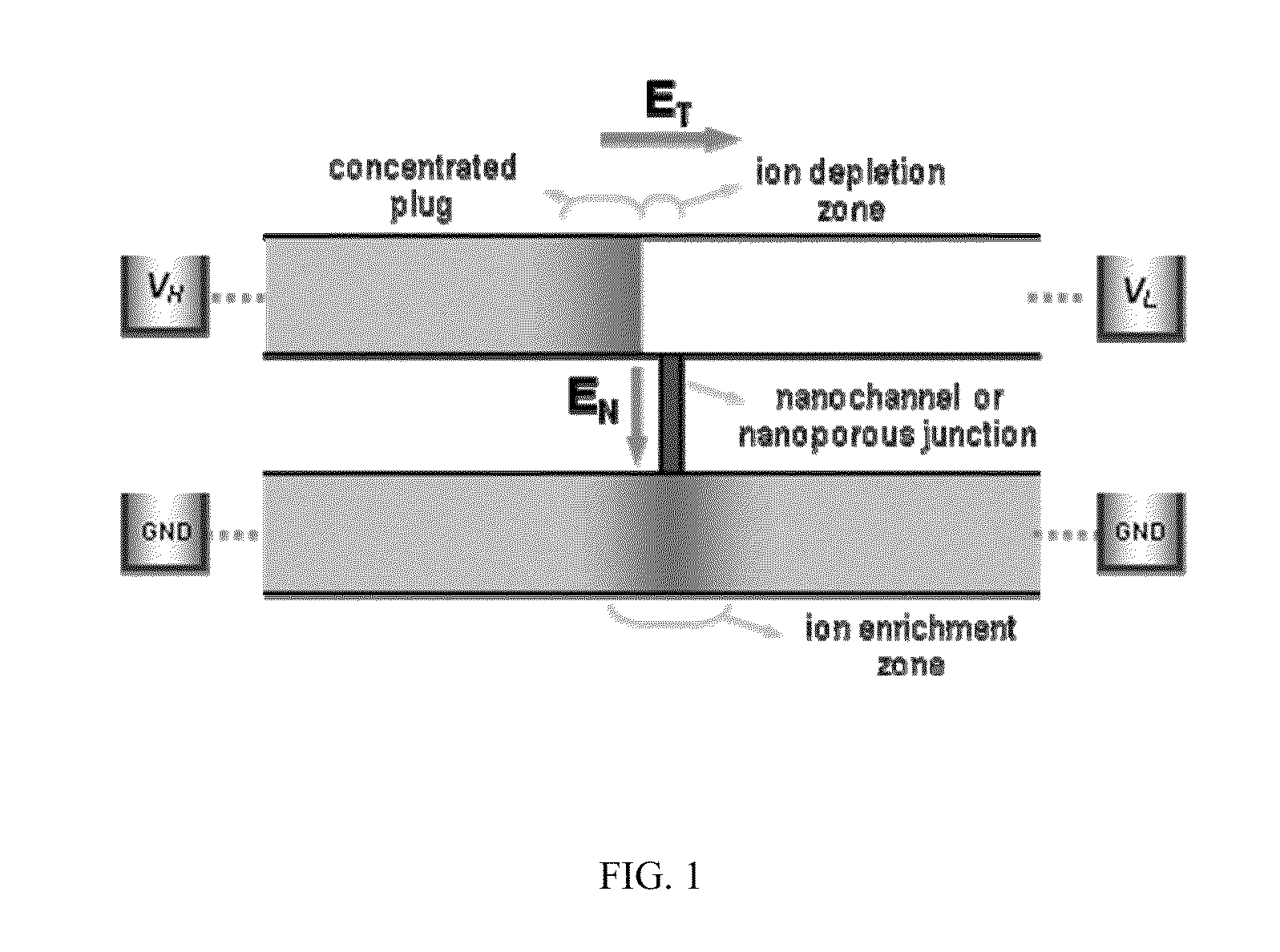
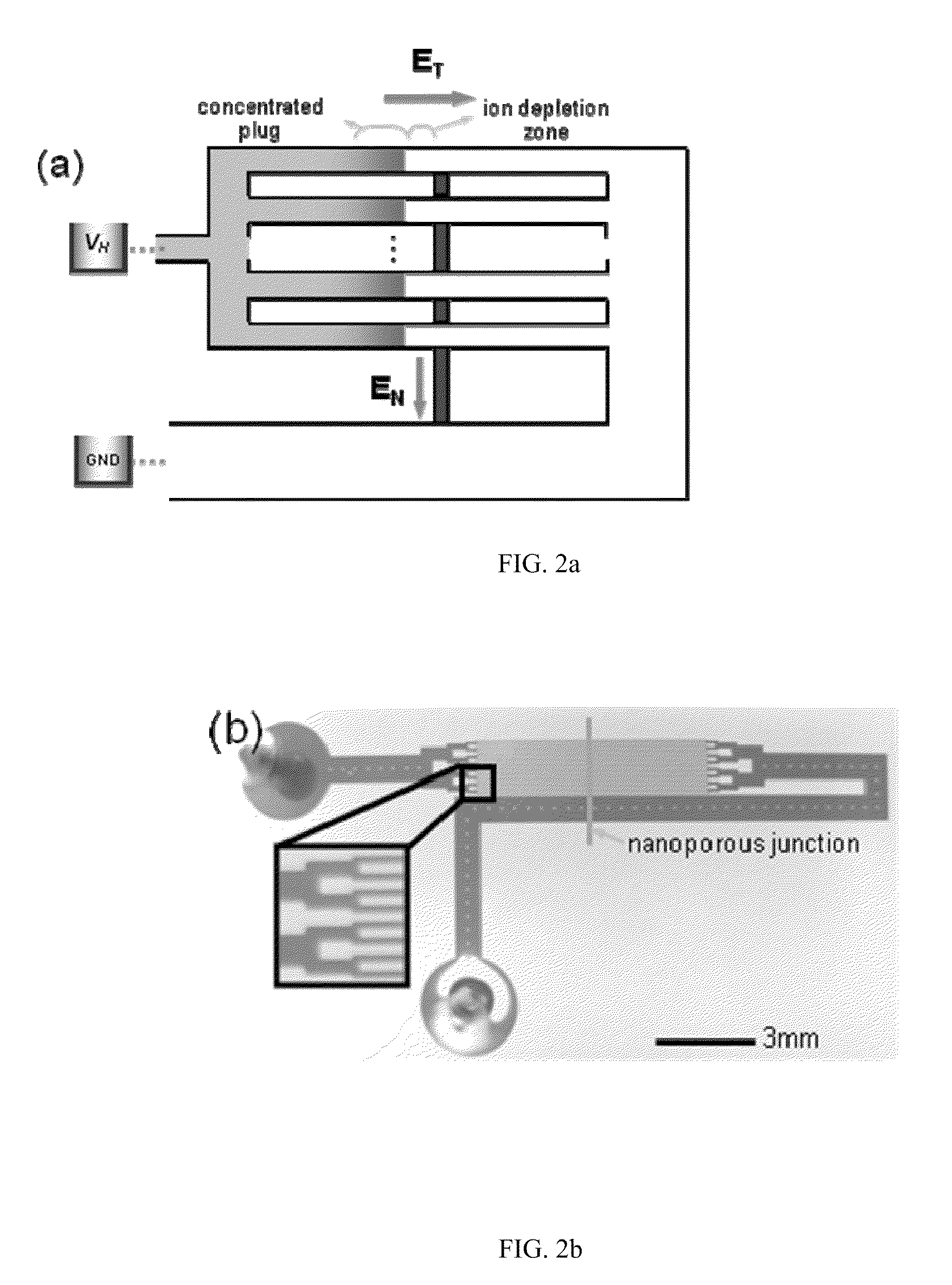
Method for Building Massively-Parallel Preconcentration Device for Multiplexed, High-Throughput Applications
InactiveUS20110220498A1Reduce needLow efficiencySludge treatmentCircuit elementsMultiplexingEngineering
A multiplexed concentration interface that can connect with a plurality of microchannels, conventional 96 well plates or other microarrays is disclosed. The interface can be used in biosensing platforms and can be designed to detect single or multiple targets such as DNA / RNA, proteins and carbohydrates / oligosaccharides. The multiplexed concentration device will provide a set of volume-matched sample preparation and detection strategies directly applicable by ordinary researchers. Furthermore, a multiplexed microfluidic concentrator without buffer channels is disclosed.
Owner:MASSACHUSETTS INST OF TECH
Indirect ELISA kit for detecting African swine fever virus
The invention discloses an indirect ELISA kit for detecting African swine fever virus (ASFV) antibody, and a use thereof, and belongs to the technical field of biology. The kit is characterized by: adopting prokaryotic expressed recombined P54 protein as coating antigen; detecting antibody against to the ASFV in swine serum according to an indirect ELISA principle. The coating antigen in 96-well plates of the kit is the prokaryotic expressed recombined P54 protein which has good antigenicity. The enzyme-linked immunospecific assay kit provided by the present invention comprises the 96-well plates coated by the P54 protein, positive control, negative control, horseradish peroxidase-labeled rabbit anti-porcine IgG polyclonal antibody, a concentrated cleaning solution, a serum dilution, a TMB substrate indicator and a stop buffer. The kit provided by the present invention can be provided for screening mass samples. In addition, the main reagents in the kit are provided in a working fluid manner such that the reagents are used conveniently.
Owner:陈文刚
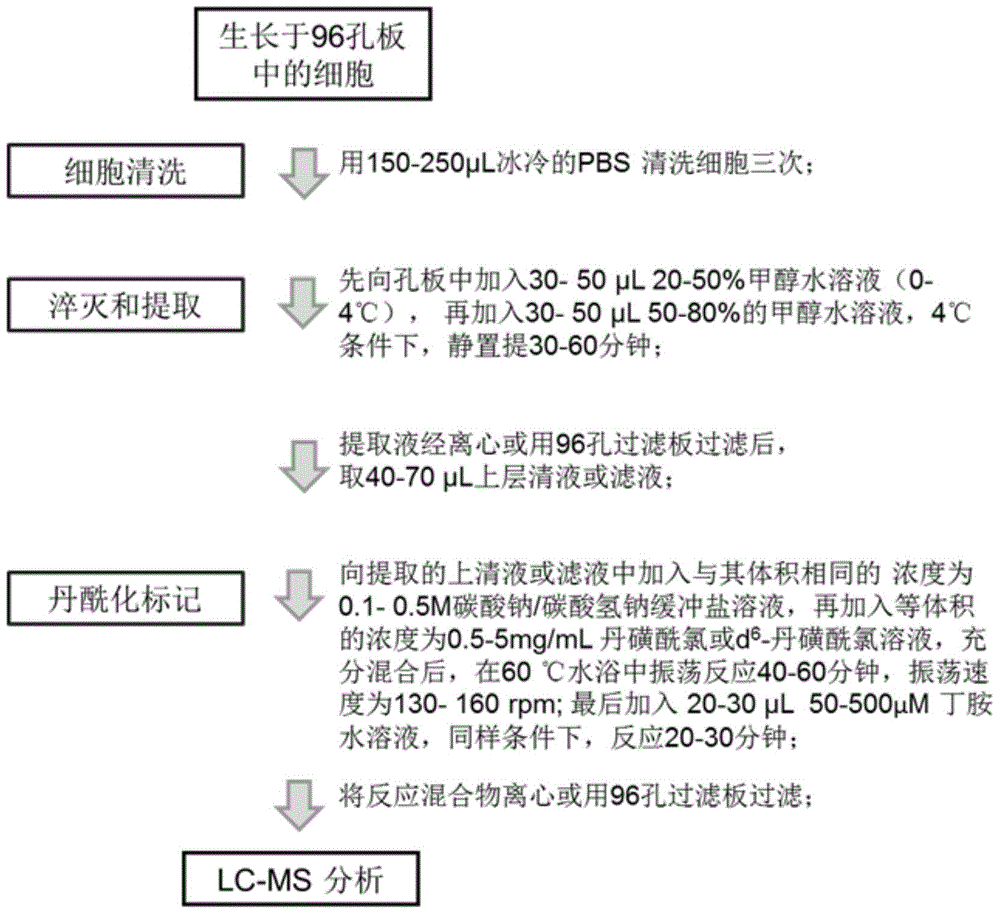
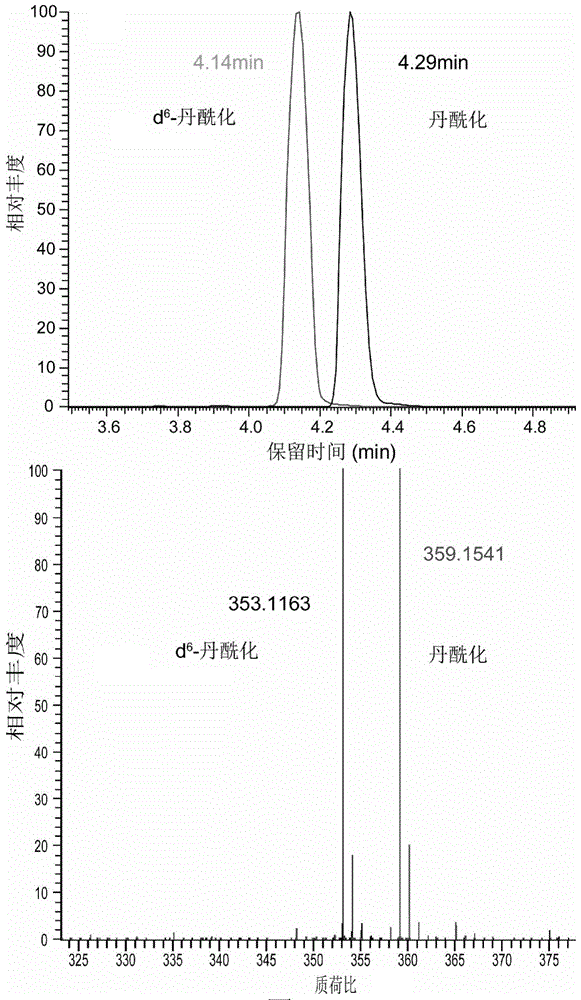
Intracellular amino acid metabolic profiling analysis method
InactiveCN105527350AThe pre-processing process is simpleImprove ionization efficiencyComponent separationDerivatizationMetabolome
The invention discloses an intracellular metabolic profiling high-flux analysis method of a few of cells (with the number of 103-104). The method comprises simple and efficient cell quenching and metabolite extraction, metabolite derivatization and mass spectrometric detection. The intracellular metabolic profiling high-throughput analysis method has the characteristics of simpleness, fastness, high sensitivity and good repeatability and is suitable for high-throughput analysis of metabolome of cells cultured through a 96-well plate and cells with a low yield, wherein the analysis fields comprise drug large-scale screening, evaluation and stem cell research.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Chemiluminescence detection kit of swine foot-and-mouth disease 3ABC and 2C antibodies
ActiveCN106596932AHigh purityDetection of high sensitivityChemiluminescene/bioluminescenceEscherichia coliSerum ige
The invention discloses a chemiluminescence detection kit of swine foot-and-mouth disease 3ABC and 2C antibodies and belongs to the field of immunological detection. The kit comprises a chemiluminescent immunoreaction plate, an enzyme-labeled antibody, a serum diluent, a chemiluminescent substrate, a chemiluminescent enhancer and a PBST washing liquid, and is characterized in that the chemiluminescent immunoreaction plate is a milky white opaque polystyrene 96-well plate, the bottom in each one of the wells is coated with a 3ABC-2C fusion protein, the enzyme-labeled antibody is a HRP-rabbit anti-pig lgG antibody, the serum diluent comprises Tween-20, bovine serum albumin and a lysate of escherichia coli, the chemiluminescent substrate comprises luminol and bovine serum albumin, and the chemiluminescent enhancer comprises IPP, H2O2 and Tween-20. Compared with CLIA only coated with a 3BAC antibody and the commercial common ELISA kit, the chemiluminescence detection kit has higher sensitivity, specificity and diagnostic ability and good repeatability and stability.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method for culturing lung cancer stem cells under 3D culture conditions
The invention discloses a method for culturing lung cancer stem cells under 3D culture conditions and belongs to the field of cell culture methods. The method comprises the following steps of placing human lung adenocarcinoma cell line A549 in an incubator for culturing with a RPMI-1640 complete culture medium containing 10% fetal calf serum, taking a cell suspension in a logarithm growth period, adjusting the concentration of the cells to 5*10<4>cells / ml and adding 200 mu L / well into a 96-well plate in which 50 mu L / well BME is spread; on the third day, changing the culture medium for the A549 cells with a RPMI-1640 complete culture medium containing IGF-1 and FGF and further culturing; digesting BME through a proteolytic enzyme, recovering the A549 cells from a recovery liquid and finally identifying the cells. By such design, the A549 cells form cloned mass similar to in-vivo tumor mass in vitro, which is closer to the three-dimensional growth state in the human body and thus the stable separation and effective amplification of stem cells are achieved. The cancer stem cells capable of resisting anticancer drugs are screened.
Owner:TIANJIN MEDICAL UNIV CANCER INST & HOSPITAL
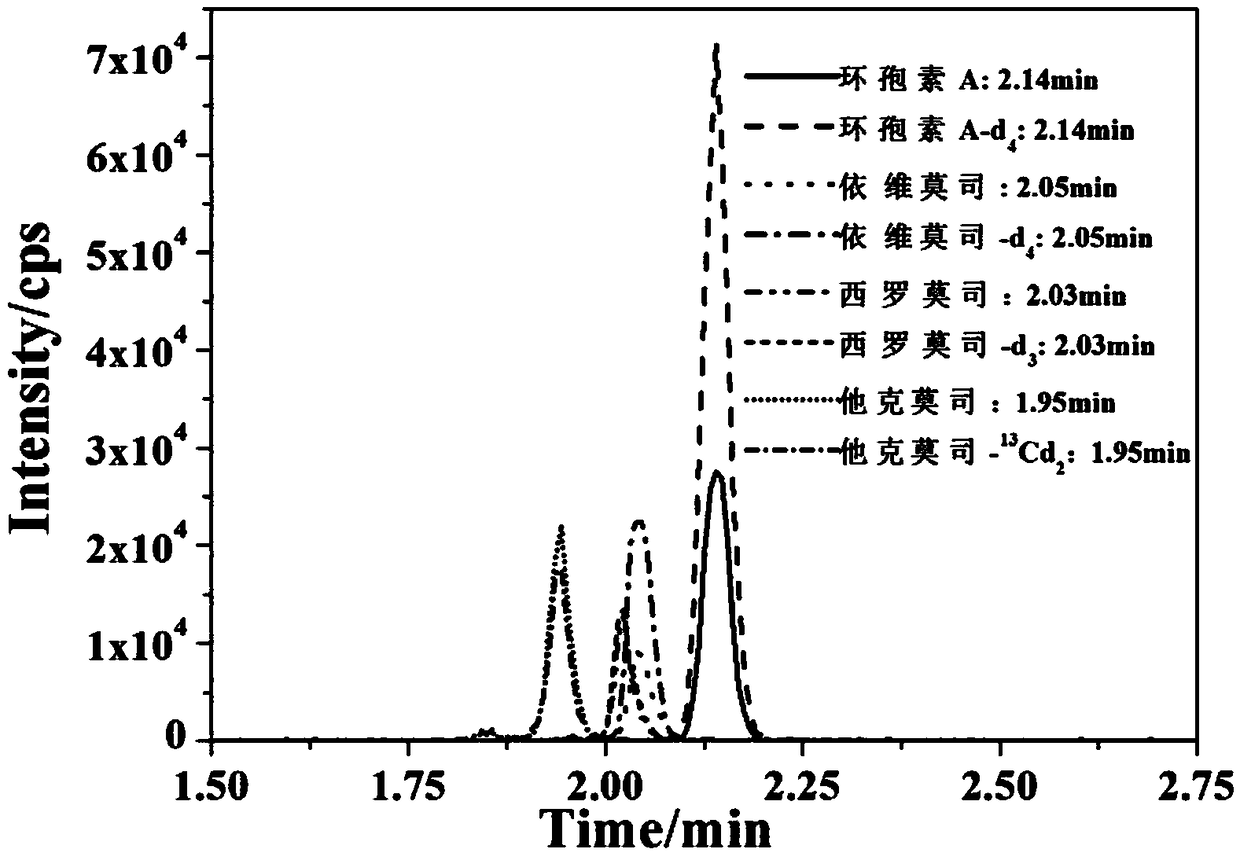
Kit and detecting method for accurately measuring concentration of four immunosuppressant drugs in human whole blood
InactiveCN109187839ASmall sample sizeHigh detection specificityComponent separationEverolimusPretreatment method
The invention provides a kit for accurately measuring the concentration of four immunosuppressant drugs in human whole blood. The kit comprises the calibrators of tacrolimus, sirolimus, everolimus andcyclosporine A, the internal standards of the tacrolimus-13Cd2, the sirolimus-d3, the everolimus-d4 and the cyclosporine A, the quality control serums of the tacrolimus, the sirolimus, the everolimusand the cyclosporine A, sample treatment liquid, a 96-well plate assembly, and a mobile phase. According to the kit and detecting method for accurately measuring the concentration of four immunosuppressant drugs in human whole blood, a clean treatment liquid can be obtained through a simple 96-well plate protein precipitation pretreatment method without complex purification steps, and the required sample amount is small and can be used together with a liquid chromatography-tandem mass spectrometry; and four different immunosuppressant drugs can be detected at the same time, that is, the single-sample multi-index synchronous detection can be realized, the detection specificity is good, the sensitivity is high, the whole detection time is short, the throughput is high, and the cost is low.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
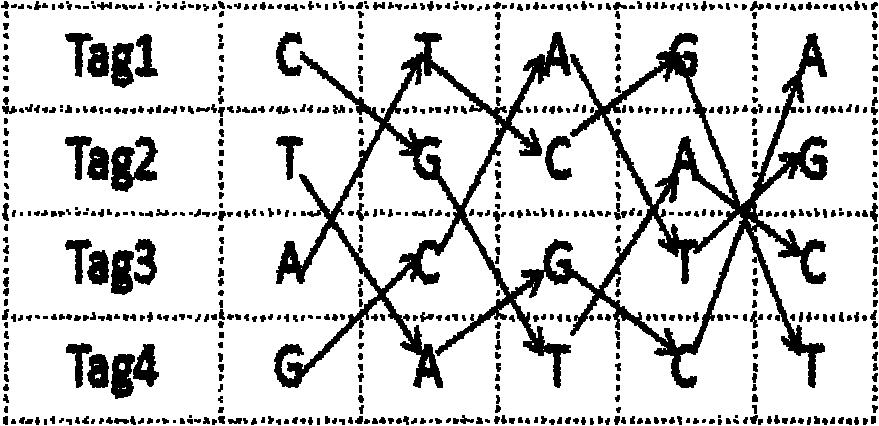
Nucleic acid label for second-generation high-flux sequencing and design method thereof
InactiveCN102115789ALow costNo loss of reliabilityMicrobiological testing/measurementHigh fluxBiology
The invention discloses a nucleic acid label for second-generation high-flux sequencing and a design method thereof, and relates to a nucleic acid label. The invention provides the nucleic acid label for second-generation high-flux sequencing and the a design method thereof, which are capable of quickly, efficiently, specially and simultaneously labeling almost one hundred independent samples, applicable to the mixed sequencing of a plurality of samples and used under the condition with a 96-pore plate. The nucleic acid label comprises an A group including eight labels with the length of 5nt,a B group including eight labels with the length of 5nt and a C group including twelve labels with the length of 6nt. Designing a first tag sequence: Tag 1: CTAGA; designing other three tags: Tag 2: TGCAG; Tag 3: ACGTC; Tag 4: GATCT; designing other four tags: Tag 5: CGTAC; Tag 6: TAGCA; Tag 7: ATCGT; Tag 8: GCATG; and calculating a positional number with an identical basic group between every two tags.
Owner:XIAMEN UNIV
Kit for extracting DNA for sampling in minute quantities without wound, and method for extracting DNA
InactiveCN101050414AAvoid churnAvoid pollutionSugar derivativesMicrobiological testing/measurementHigh fluxSilica gel
This invention discloses test kit and method for extracting DNA by noninvasive micro sampling. The test kit contains lysate solution, silica gel adsorber, cleaning solution, and eluant. The content and quality of DNA product extracted with the test kit can satisfy the following operation such as PCR and sequencing. The test kit is operated in single-tube form to prevent the loss of DNA component, and contamination during transfer. Besides, the test kit can be used together with 96 hole plate for high-flux extraction due to its in situ extraction mode. The test kit has such advantages as easy operation, low time consumption, high efficiency, and high quality and content of extracted product.
Owner:FUDAN UNIV
Compliant surface multi-well culture plate
InactiveCN101842474ABioreactor/fermenter combinationsBiological substance pretreatmentsCollagen iEngineering
A multi-well plate can be loaded with a range of compliant substrates. Commerically-available assays can be used to test cellular responses across a plate with shear modulus from 50 to 51200 Pascals. Cells can be grown in the plates, and can be manipulated and analyzed. Hydrogels can be attached to the bottom of a well. The plates can support the attachment and growth of different cell types and can be compatible with standard 96-well and 384-well plate assays. The mechanical properties of the hydrogels can be reproducible and stable to increase the shelf life of the substrate. The hydrogel can be compatible with growth of a variety of cell types, various attachment ligands such as collagen I, collagen IV, flbronectin, vitronectin, laminin, or RGD peptides and can be coupled to the gel surface.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Micro-scale cell electro-transfection micro-fluidic chip, electro-transfection sorter and applications thereof
PendingCN107988070AGuaranteed qualityLow costBioreactor/fermenter combinationsBiological substance pretreatmentsElectricityInjection port
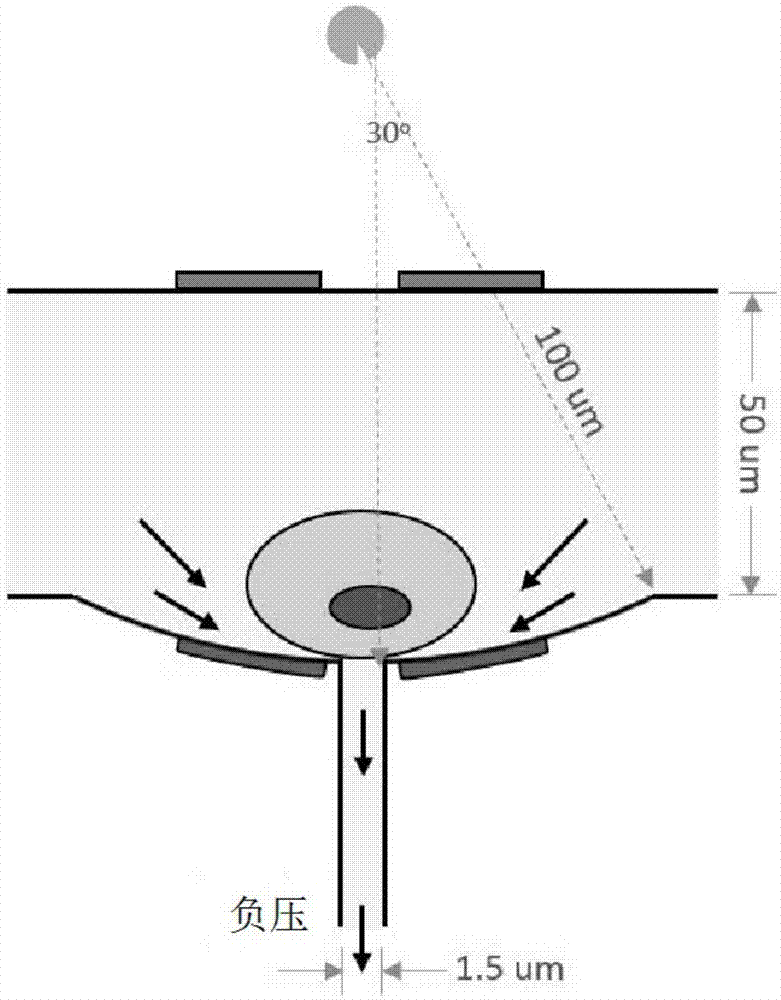
The invention relates to a micro-scale cell electro-transfection micro-fluidic chip, an electro-transfection sorter and applications thereof. The micro-scale cell electro-transfection sorter comprisesan electro-transfection unit, a display screen, an exterior box, a power supply unit, a micro control unit, and a main sensor. The electro-transfection unit comprises the chip. The display screen isused to send a command to the micro control unit and receive and display feedback information from the micro control unit and the main sensor. The micro control unit is used to receive the command sent by the display screen and control the electro-transfection unit and the power supply unit. The electro-transfection unit is used to complete cell transfection. The main sensor is used to receive feedback information from the electro-transfection unit and send the feedback information to the display screen and the micro control unit. The micro-scale cell electro-transfection micro-fluidic chip comprises an injection port, a discharge port, a negative pressure channel, a positive pressure channel, and a main channel, and a 96-well plate is arranged behind the discharge port. The provided chipcan guarantee that the transfection states in the main channel are identical during the transfection process, the transfection efficiency is guaranteed, the cell quality is guaranteed by the 96-well plate, and the subsequent cell culture becomes more convenient.
Owner:ALLIFE MEDICAL SCI & TECH CO LTD
High through-put detection of pathogenic yeasts in the genus trichosporon
InactiveUS20060216723A1Rapid and simple to performSugar derivativesMicrobiological testing/measurementSequence analysisPresent method
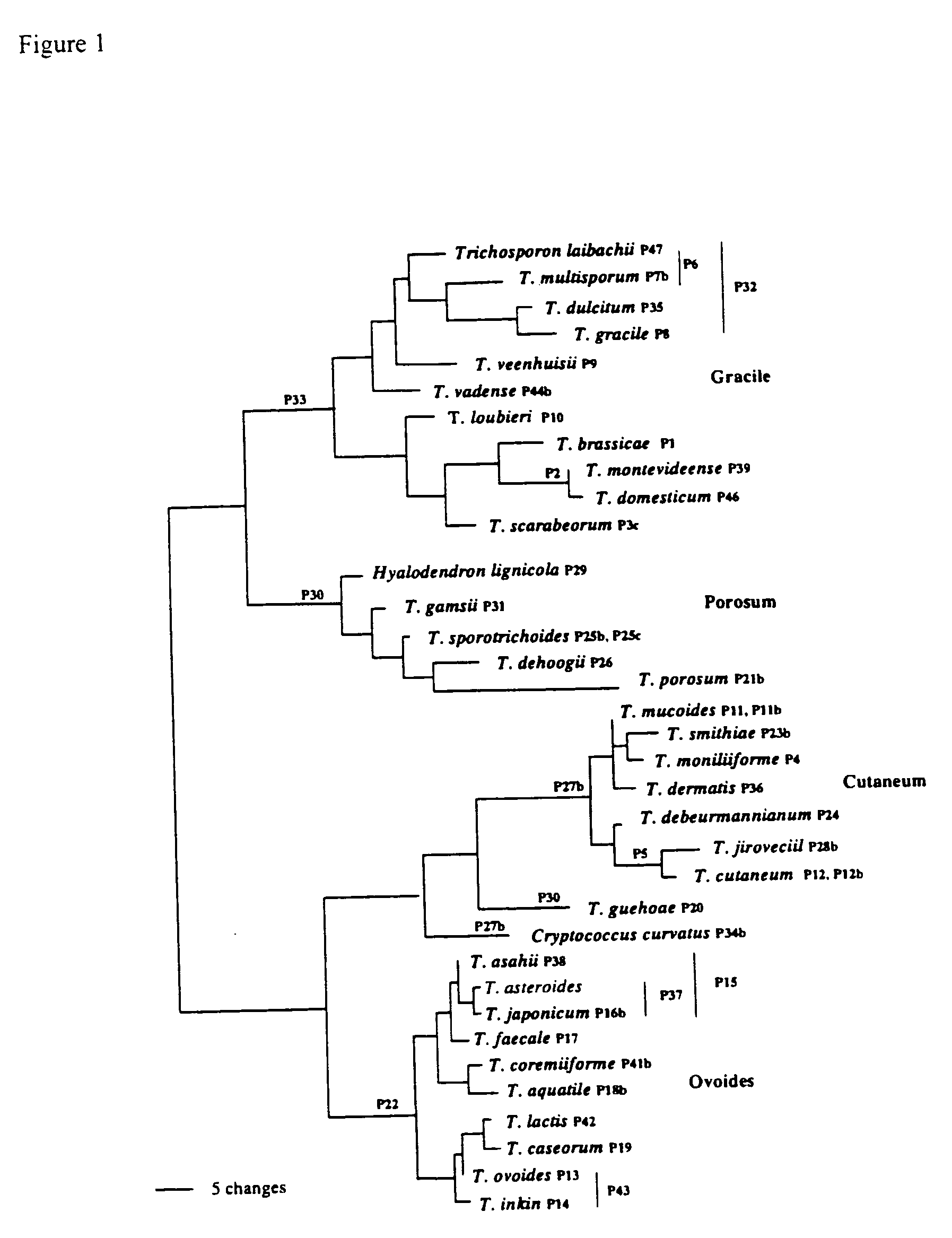
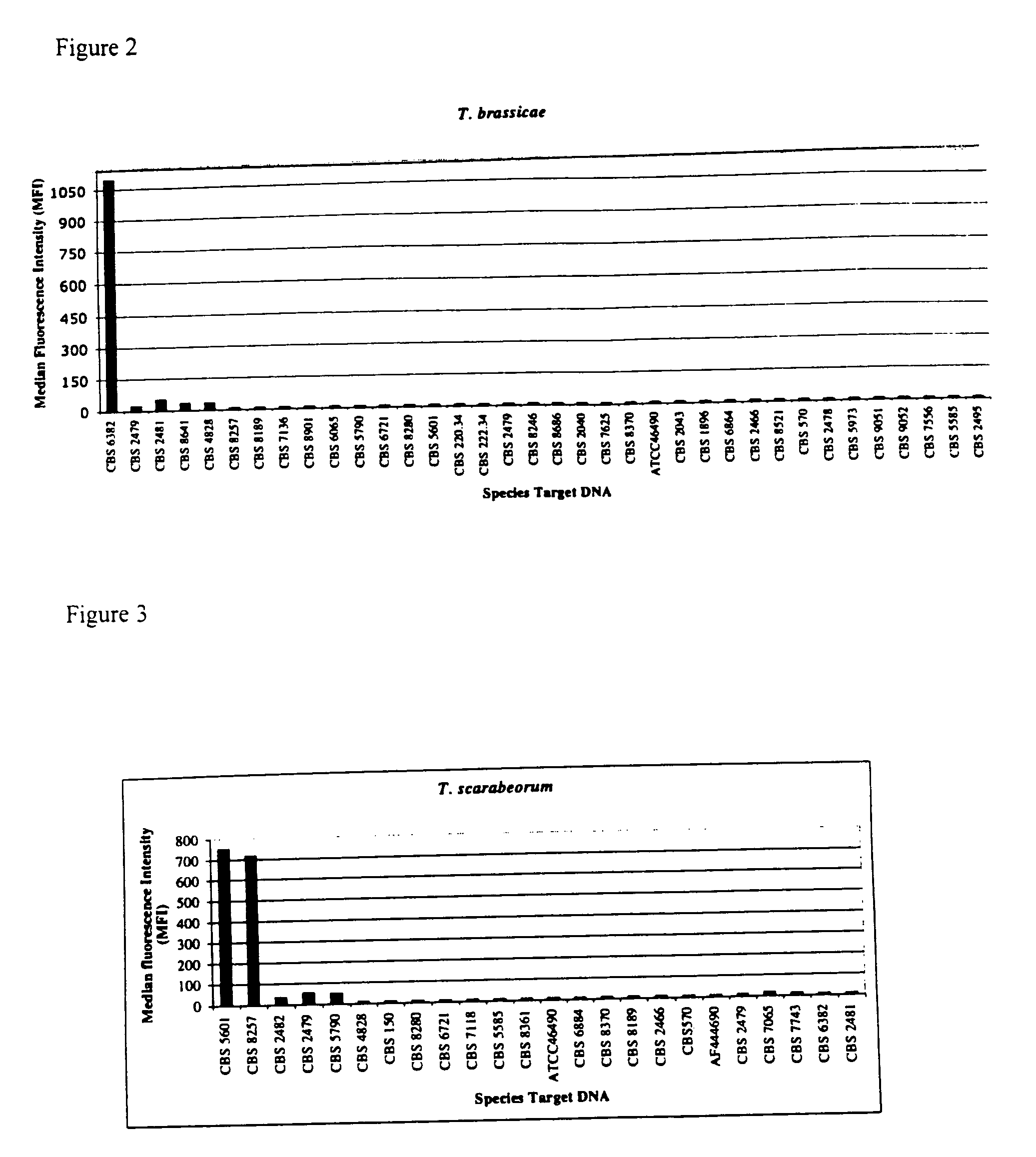
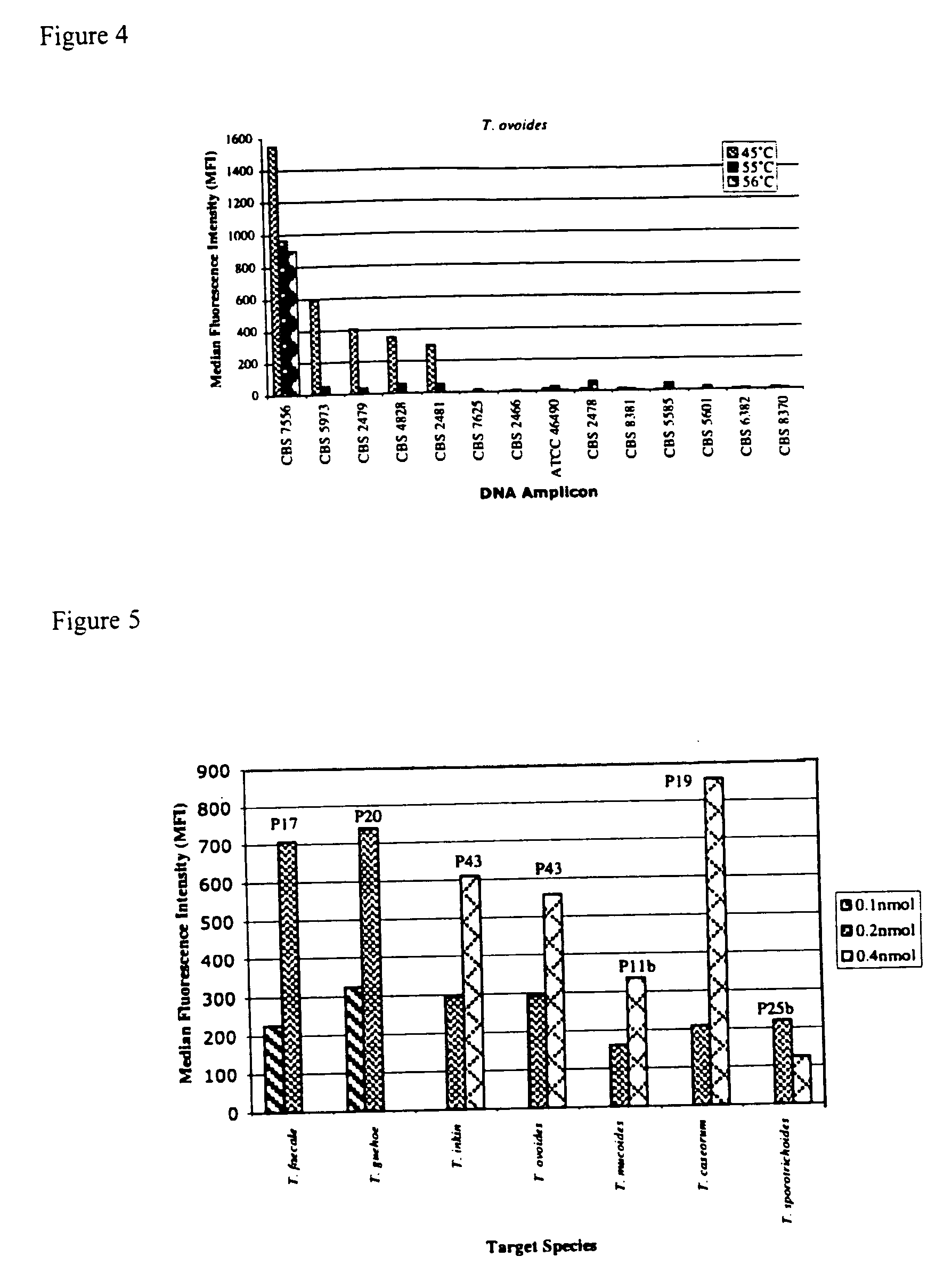
The emergence of opportunistic and antifungal resistant strains has given rise to an urgent need for a rapid and accurate method for the detection of fungal pathogens. In this application, we demonstrate the detection of medically important fungal pathogens at the species level. The present method, which is based on a nucleotide hybridization assay, consists of a combination of different sets of fluorescent beads covalently bound to species specific capture probes. Upon hybridization, the beads bearing the target amplicons are classified by their spectral addresses with a 635 nm laser. Quantitation of the hybridized biotinylated amplicon is based on the fluorescent detection with a 532 nm laser. Using this technology we designed and tested various multiplex formats, the performance of forty eight species specific and group specific capture probes designed from sequence analysis in the D1 / D2 region of ribosomal DNA, internal transcribed spacer regions (ITS), and intergenic spacer region (IGS). Species-specific biotinylated amplicons (>600 bp) were generated with three sets of primers to yield fragments from the three regions. The developed assay was specific and relatively fast, as it discriminated species differing by one nucleotide and required less than 50 min following amplification to process a 96 well plate with the capability to detect up to 100 species per well. The sensitivity of the assay allowed the detection as low as 102 genome molecules in PCR reactions and 107 to 108 molecules of biotinylated amplification product. This technology provided a rapid means of detection of Trichosporon species and had the flexibility to identify species in a multiplex format by combining different sets of beads. The assay can be expanded to include all known pathogenic fungal species.
Owner:MIAMI UNIVERISTY OF
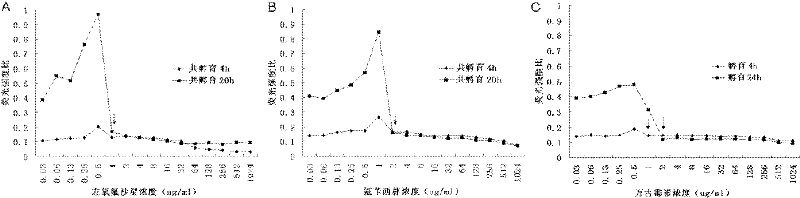
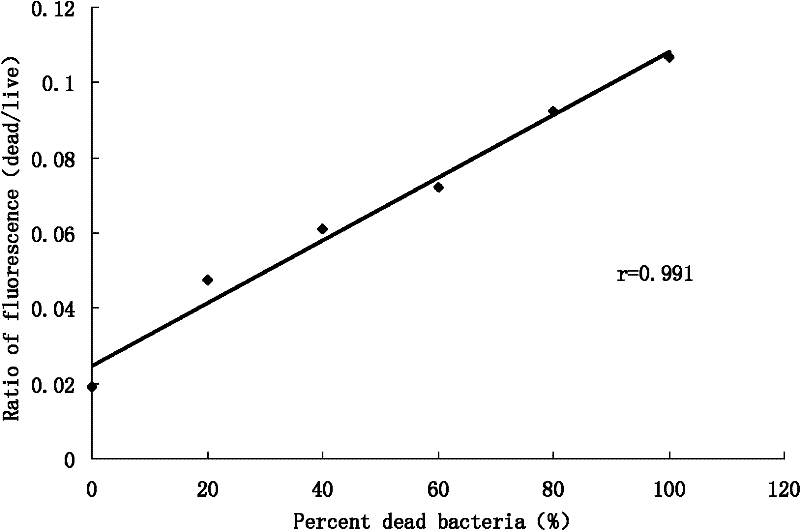
Method for Determination of Minimum Inhibitory Concentration of Drugs
ActiveCN102288586AMIC value is accurateLow costFluorescence/phosphorescenceMinimum inhibitory concentrationConcentration gradient
The invention discloses a method capable of rapidly, simply, conveniently and quantitatively detecting minimal inhibitory concentration, and the method comprises the following steps of: in accordance with the standard of CLSI (Clinical and Laboratory Standards Institute) (Version 2010), adding a fresh enterococcus suspension of a certain concentration into a sterile 96-well plate containing concentration gradient antibacterials for co-incubation; upon the ending of the 4-hour incubation, adding a fixed amount of fluorescent dyes SYTOX Green and DAPI (4,6-diamino-2-phenyl indole) to all the wells, protecting the wells from light for 15 minutes at room temperature, reading the fluorescent intensity of the two dyes in the wells by use of a fluorescent microplate reader respectively; after relevant background fluorescence is deducted, drawing a corresponding curve between bacterial fluorescence intensity ratio (Pdead / livel) and concentration of drug (CDrug), and determining the minimal concentration of drug corresponding to the moment the Pdead / livel is no longer fluctuated as the MIC (Minimal Inhibitory Concentration) of the antibiotic to the bacterium. The detection method provided by the invention has the characteristics of being simple, convenient, rapid and objective and being capable of performing mathematic statistics and analysis directly on detected data, becomes a new detection method for determining the minimal inhibitory concentration of antibacterials, and has a wide application prospect.
Owner:BEIJING FRIENDSHIP HOSPITAL CAPITAL MEDICAL UNIV
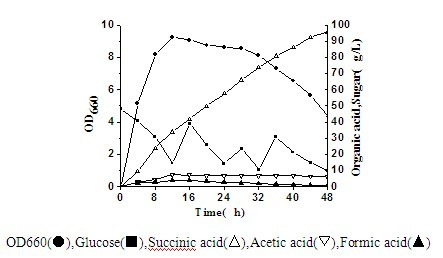
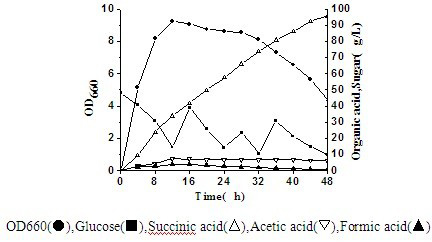
Actinobacillus succinogenes capable of producing succinic acid
ActiveCN102533622AReduce manufacturing costIncrease production intensityBacteriaMutant preparationSaccharic acidD-Glucaric Acid
The invention discloses actinobacillus succionogenes capable of producing succinic acid with high yield and a method for screening and producing the succinic acid by a fermentation method. The actinobacillus succinogenes takes CGMCC1593 as the original strain and is obtained by blending a plurality of turns of protoplast in a progressive way through a method of '96-pore plate culture, HPLC concentration detection and then anaerobic bottle re-screening'. The actinobacillus succinogenes has already been preserved on February 26, 2012 in China Center for Type Culture Collection with the preservation number CCTCCNO: M2012036. The actinobacillus succinogenes adopts fed-batch culture in a 5 to 15 L fermentation tank, 95.6 g / L succinic acid is produced in 48 hours, the production intensity is 1.99 g / (L.h), and the saccharic acid transformation rate is 0.71 g / g. Compared with other bacterial strains at home and abroad, the actinobacillus succinogenes has the advantages of high yield and lowerproduction cost.
Owner:JIANGNAN UNIV
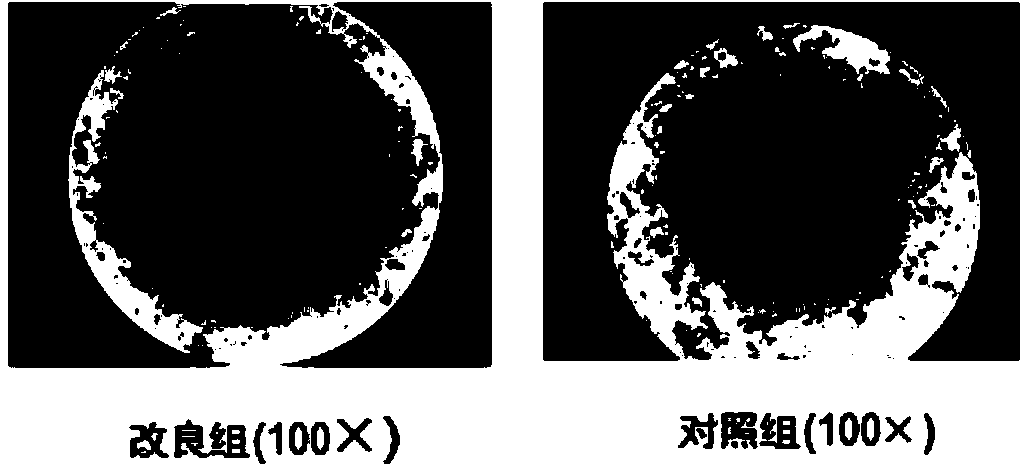
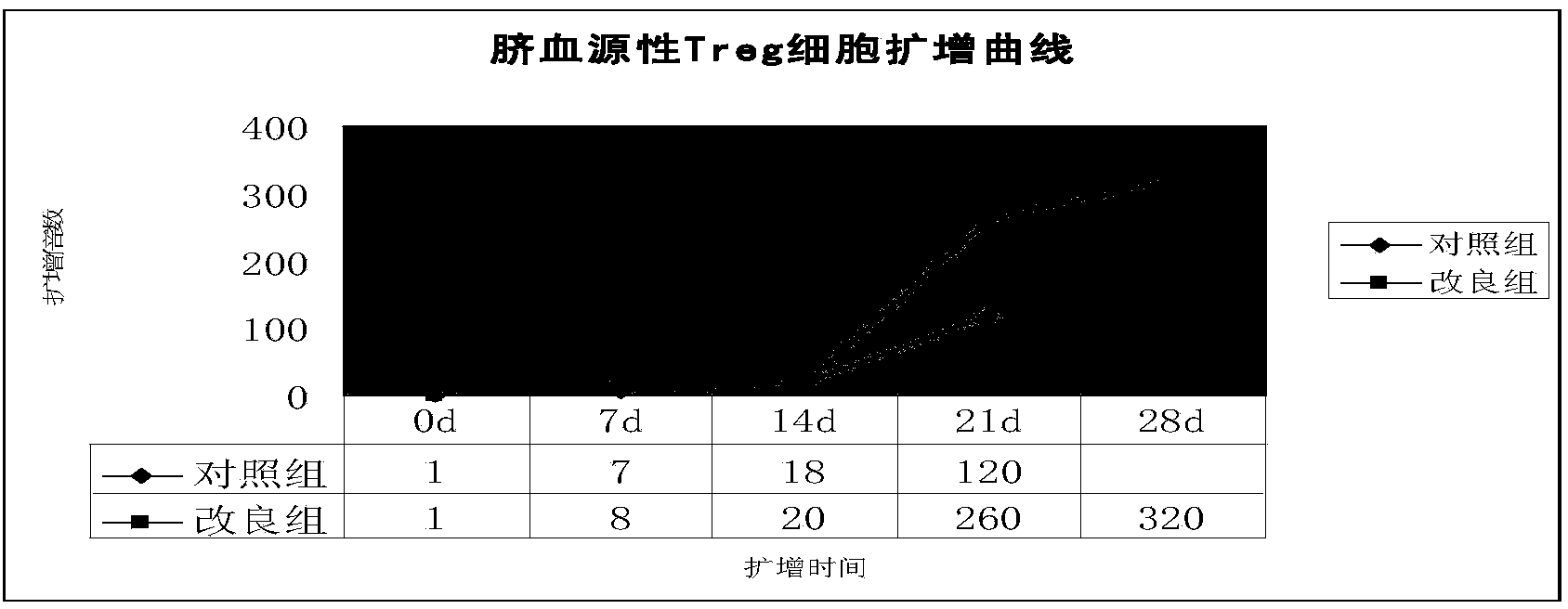
Improved expansion culture medium for regulatory T cells of human cord blood origin and application method of expansion culture medium
ActiveCN104357389AReduce riskPromote growth rateBlood/immune system cellsCulture mediumsClinical disease
The invention relates to an improved expansion culture medium for regulatory T cells of human cord blood origin and an application method of the expansion culture medium. According to the expansion culture medium, heparin anticoagulated autologous cord blood plasma accounting for 10%-12% of the volume of a culture medium, CD3-CD28 antibody co-expressed immunomagnetic beads, recombinant human interleukin 2, 2-mercaptoethanol, rapamycin, HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) and gentamicin are added into the RPMI (Roswell Park Memorial Institute)1640 culture medium; and then separated cell suspension is inoculated in a 96-well plates with a U-shaped bottom, hole-division expansion can be performed every 1-2 days, and the expansion period is 3-4 weeks. All reagents in the culture system reach the GMP (good manufacturing practice) level or are originated from autologous cord blood, so that risks caused by ingredients of animal origin are avoided, and the regulatory T cells can be used for a third-party unrelated donor and directly applied to clinical disease treatment; and compared with a traditional culture system, Treg cells (the regulatory T cells) expanded by the improved culture medium is excellent in aspects of growth speed, purity, activity, lymphocyte inhibition function and the like, and the Treg cells are expected to be used as the regulatory T cells of the third-party unrelated donor and applied to the clinical disease treatment.
Owner:HUNAN XENO LIFE SCI
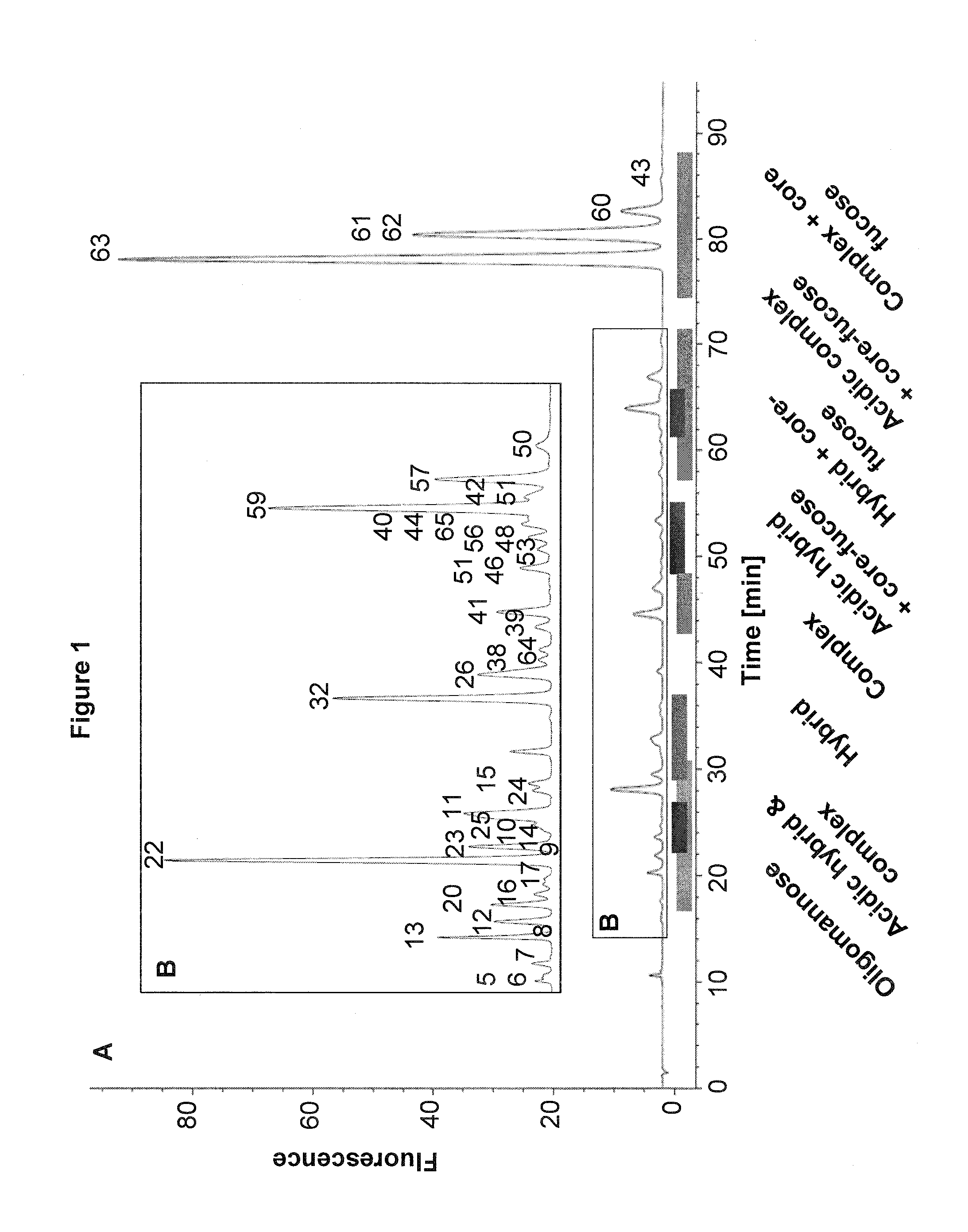
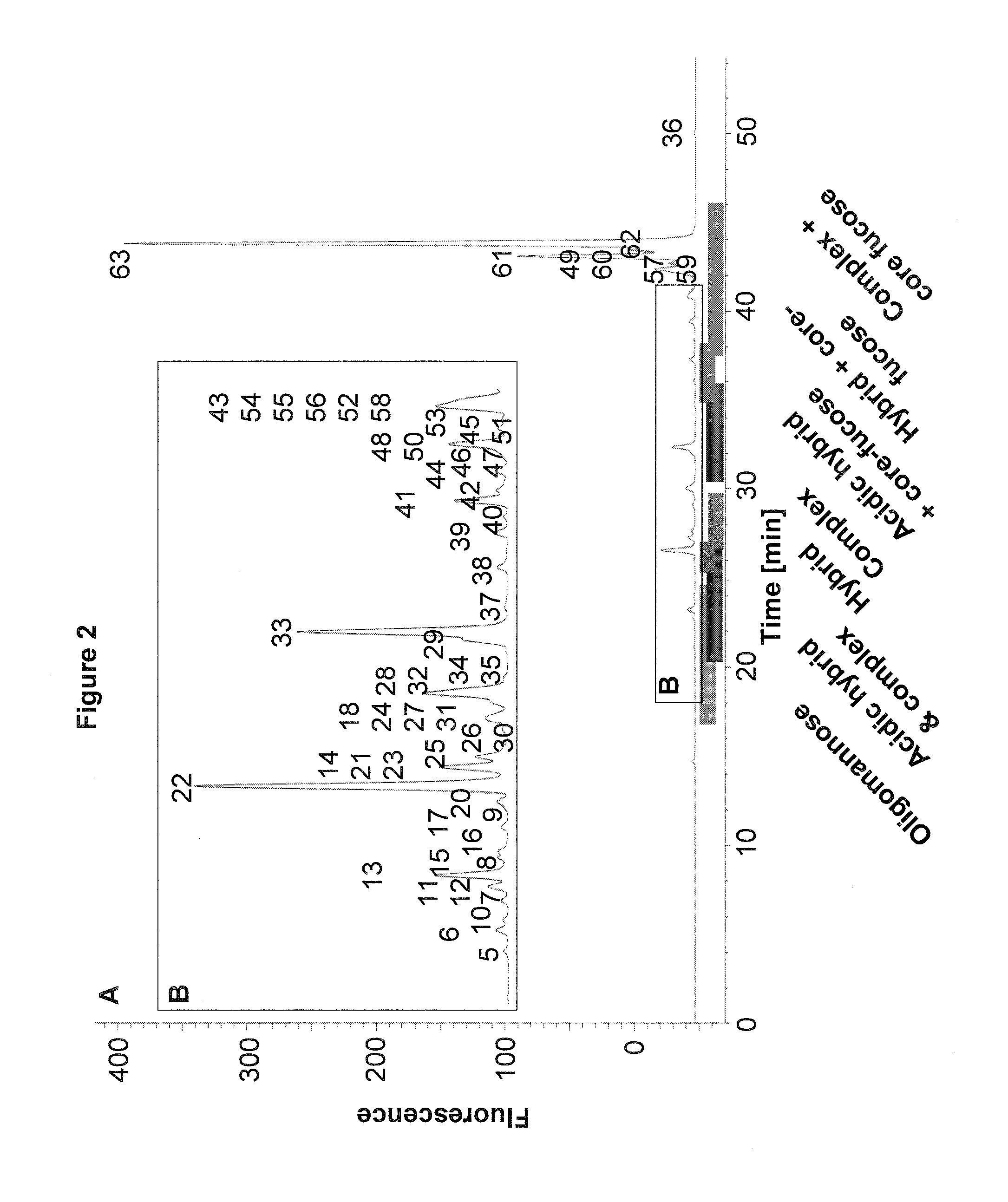
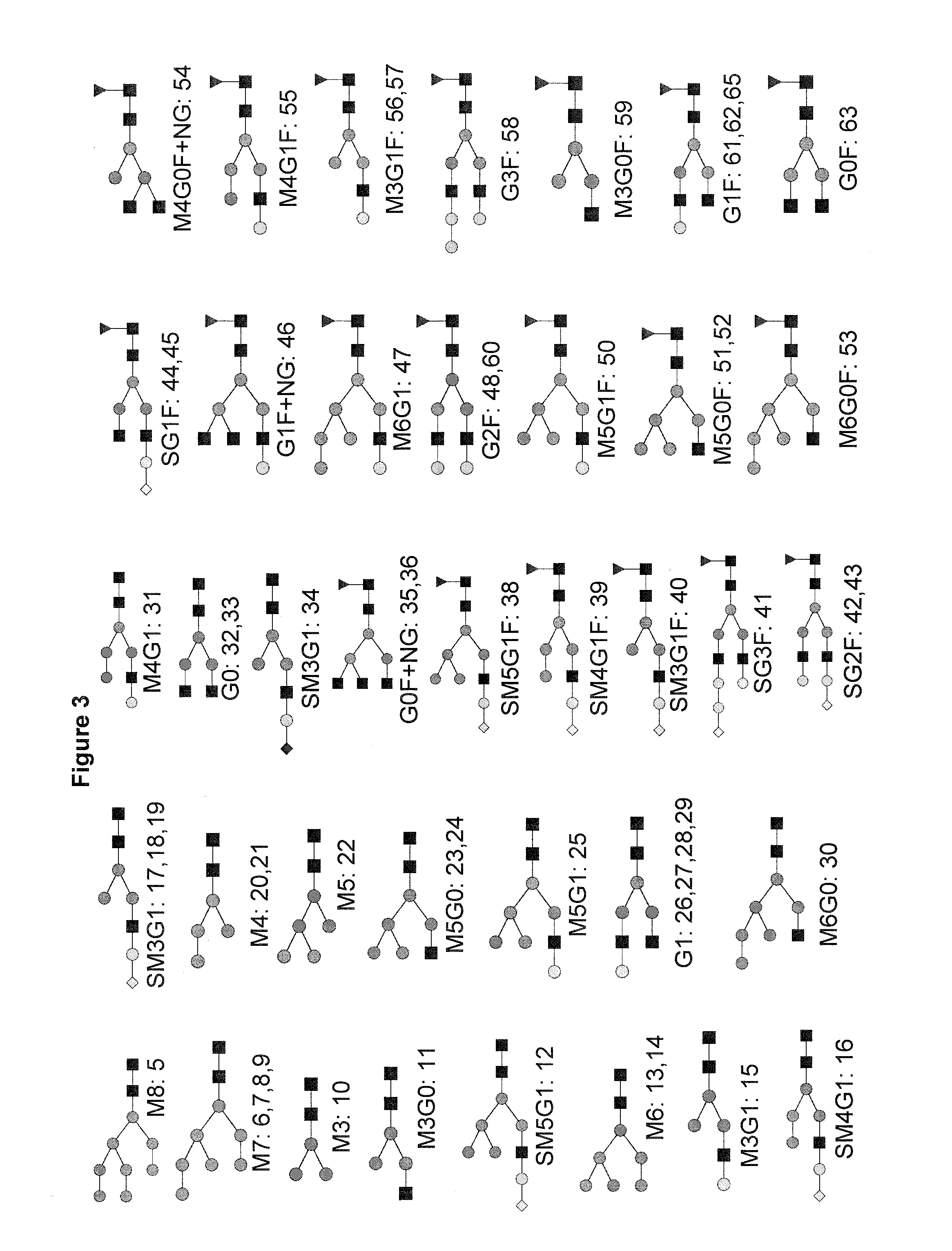
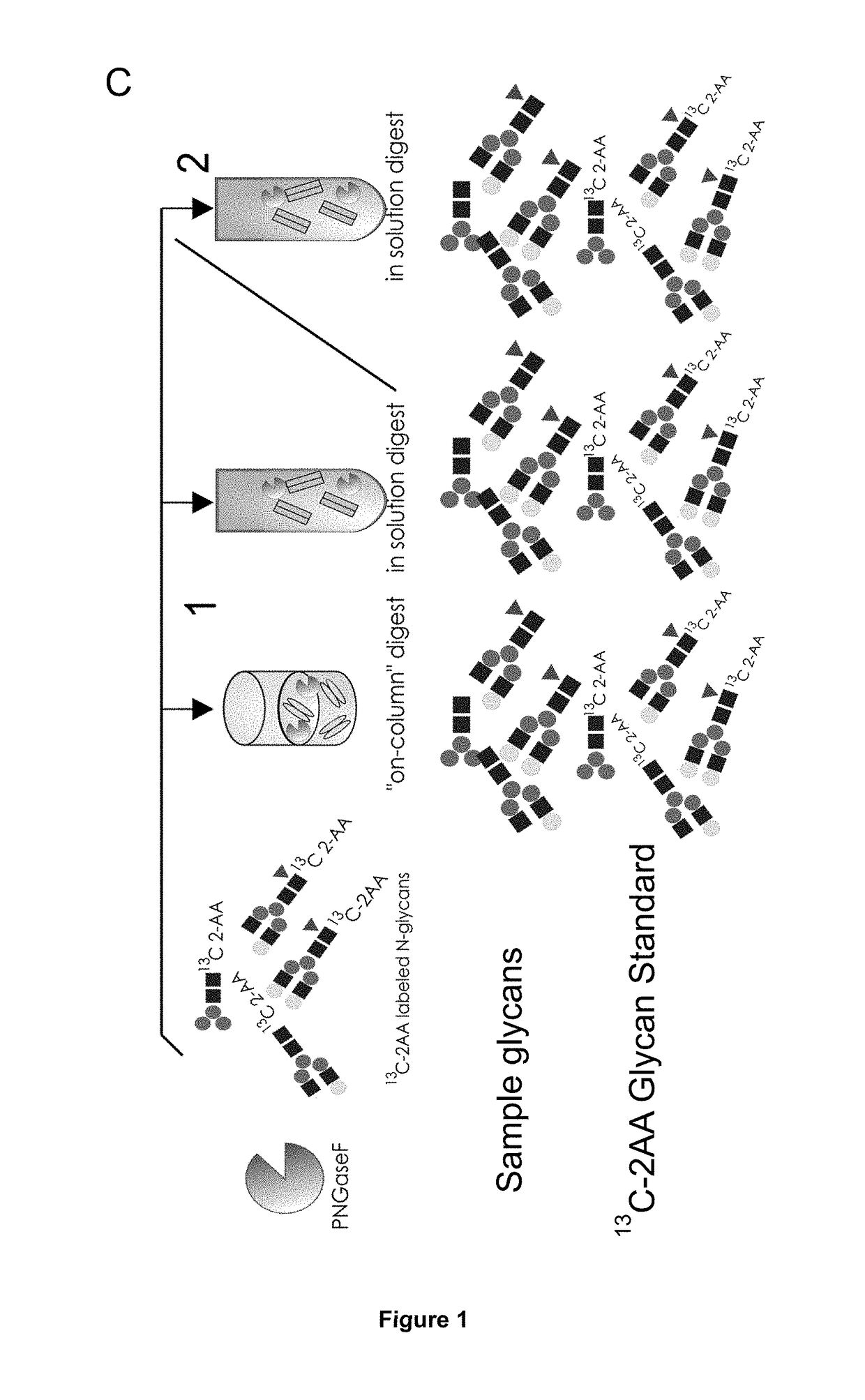
Improved method of mapping glycans of glycoproteins
InactiveUS20160018409A1High selectivityHigh sensitivityMicrobiological testing/measurementBiological testingReversed-Phase Liquid ChromatographyIon pairs
Owner:HEXAL AG
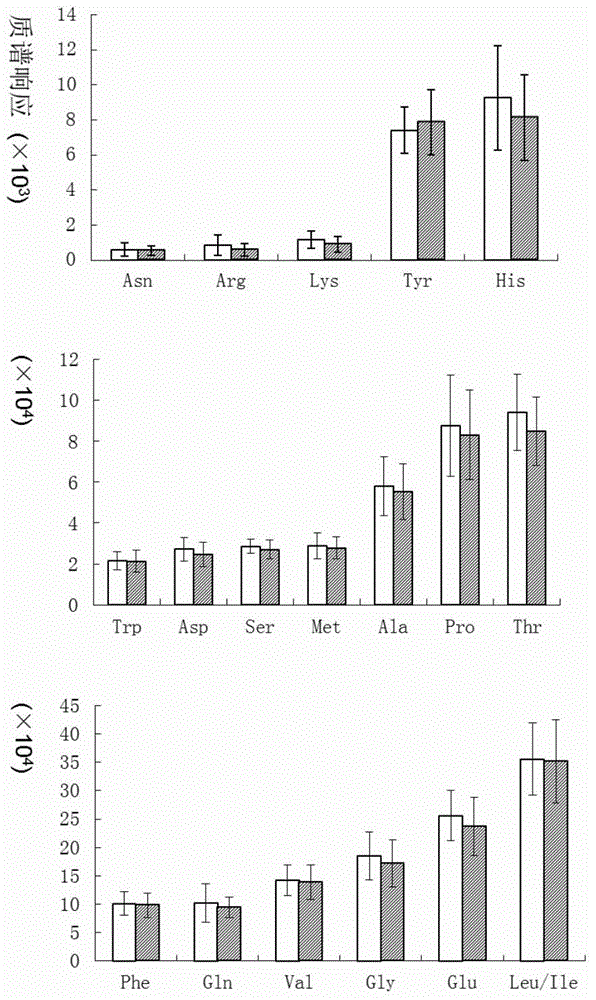
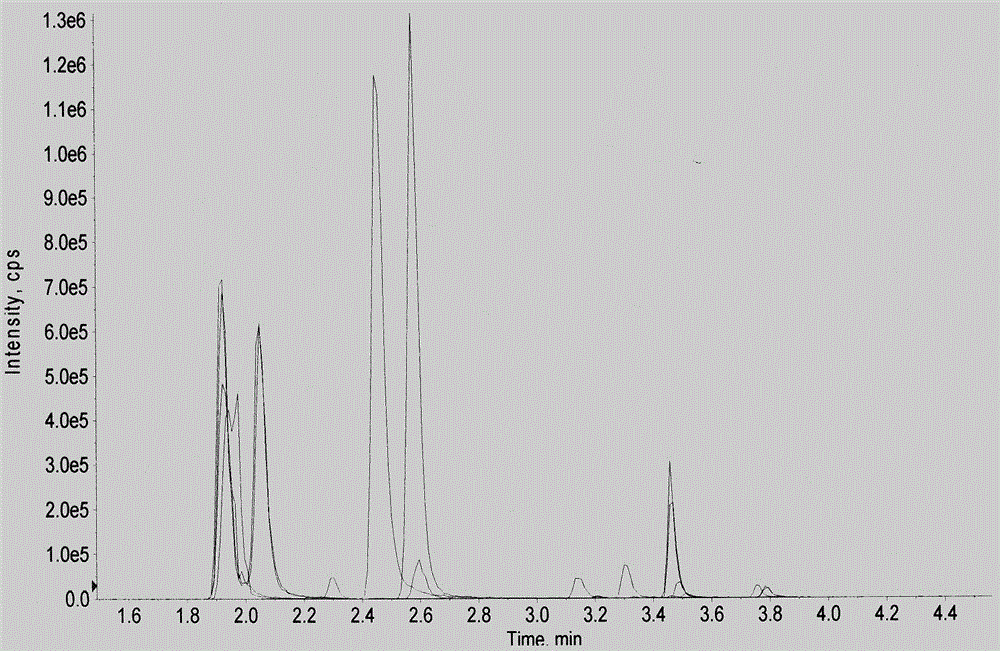
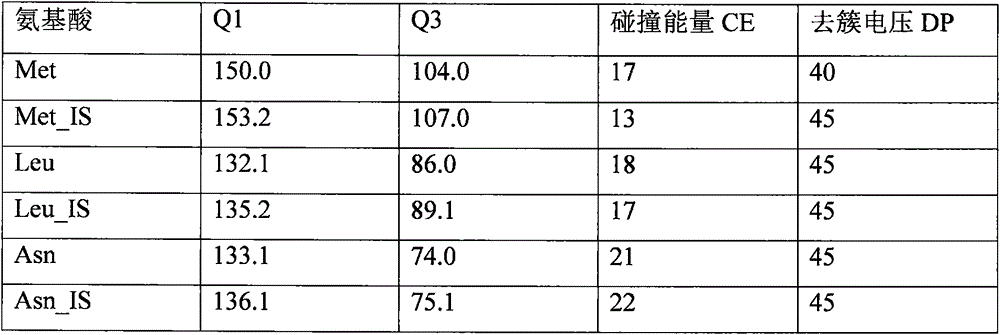
High-flux mass spectral method for detecting multiple amino acids in human body blood plasma
The invention discloses a high-flux mass spectral method for detecting multiple amino acids in human body blood plasma. The method comprises precipitating protein in blood plasma by employing an organic solvent, adding a stable isotope internal standard, performing high-speed centrifugation to extract amino acids contained in the supernatant, and directly injecting a sample to detect the multiple amino acids in the blood plasma. A 96-well plate sample pretreatment process is employed, protein precipitation, internal-standard addition and centrifugation are performed in a 96-well plate, then the 96-well plate is directly connected with an LC-MS automatic sample-injection device, 96 samples can be processed and analyzed at a time, the detection efficiency is substantially improved, also sample loss and personal error caused by repeated transfer of samples are avoided, and labor cost is reduced. At present, detection on amino acids is mainly applied to screening and diagnosis on inherited metabolic diseases, along with more and more attention of people, more and more clinic detection samples are provided. The method is a rapid high-flux detection method for detecting a large amount of clinic samples.
Owner:GUANGDONG ZHONGKEKANGYI BIOTECH CO LTD
Device for analyzing cells and monitoring cell culturing and method for analyzing cells and monitoring cell culturing using same
InactiveCN105247035AAvoid defectsReal-time analysisBioreactor/fermenter combinationsBiological substance pretreatmentsEngineeringCamera module
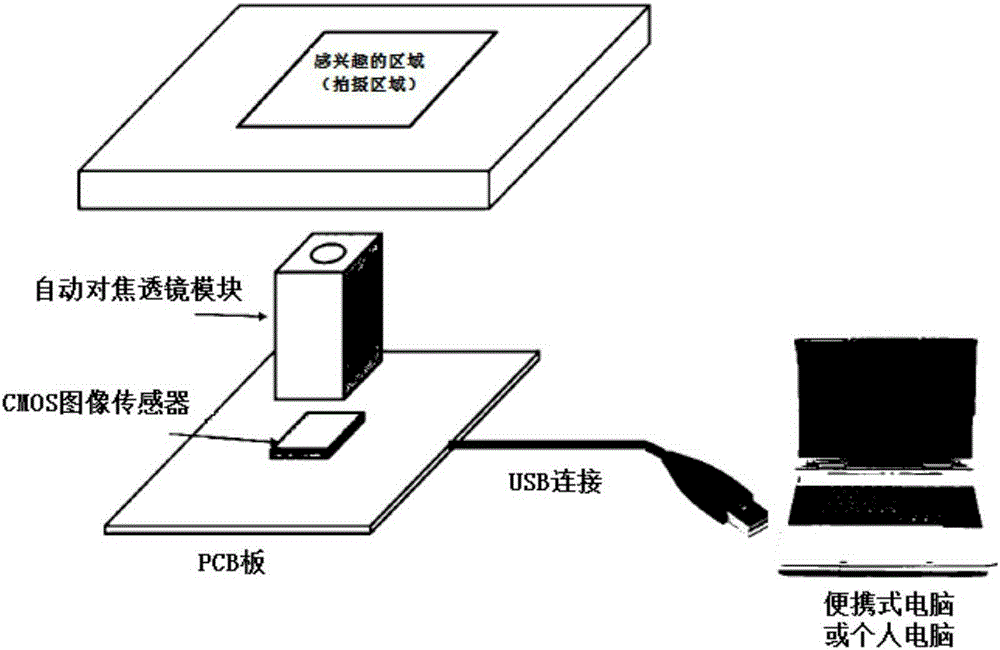
The present invention relates to a device for analyzing cells or monitoring a cell culturing process and a method for analyzing cells and monitoring cell culturing using the same. A device for analyzing cells and monitoring cell culturing according to the present invention has a camera module of MN matrix type at the lower end of a support, which contains a cell incubator, thereby making it possible to apply a multi-well cell incubator, such as a 96 well plate, and simultaneously analyze the change of various cells with respect to various culturing conditions or external environment changes, for example. Specifically, the device can selectively switch on camera modules in a specific area, thereby enabling partial observation; the device can macroscopically observe the entire well or microscopically observe an area of interest of one well at a high magnification; the device can observe the behavior image of cells in real time; the device can solve the problem of pollution while performing cell analysis and culturing; the device can remotely check the culturing state in real time.
Owner:光行科技株式会社
Extraction method of wheat genome DNA
InactiveCN104694530ASimplify the steps of DNA extractionQuality improvementDNA preparationFreeze-dryingSteel ball
The invention discloses an extraction method of a wheat genome DNA. The method comprises the following steps: putting wheat material samples to be extracted into 96-well plates respectively; then putting steel balls into each hole; uncovering and putting into a vacuum freezing dryer to be subjected to freeze drying for 20-28 hours at the temperature of -100-(-120) DEG C; grinding the wheat material samples by using a grinding instrument; finally, extracting DNA from the grinded powder samples by using a discharge gun in an improved SDS method. According to the extraction method, wheat tissue vanes are pulverized by using the grinding instrument after vacuum freeze drying is carried out; compared with a traditional liquid nitrogen grinding method, the extraction method has the advantages that the labor force is greatly released, and the working efficiency is improved; meanwhile, by using 96-well plates and the discharge gun, the sample extraction efficiency is greatly improved. The extraction method of the wheat genome DNA is convenient, fast, high in flux and high in quality.
Owner:NORTHWEST A & F UNIV
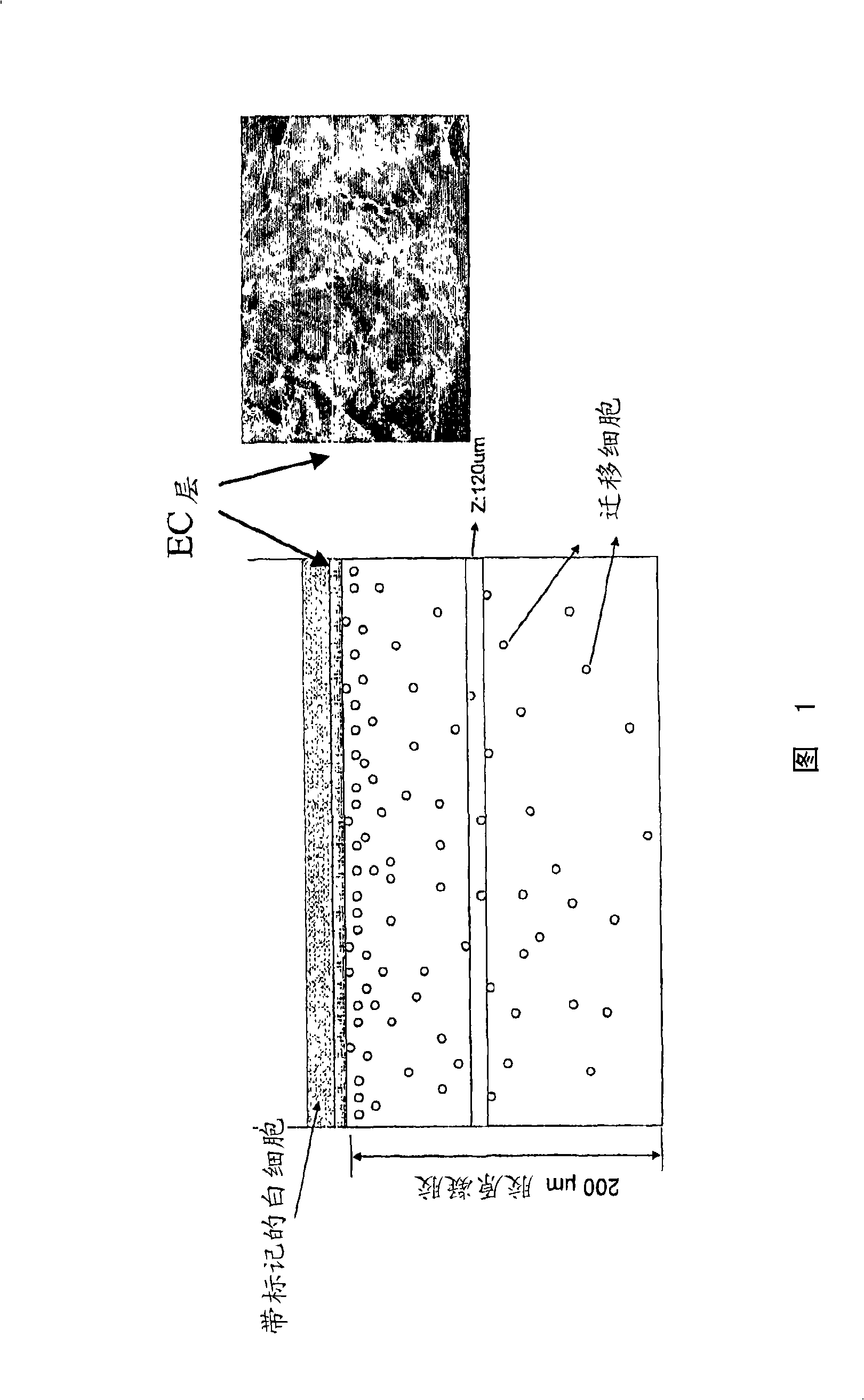
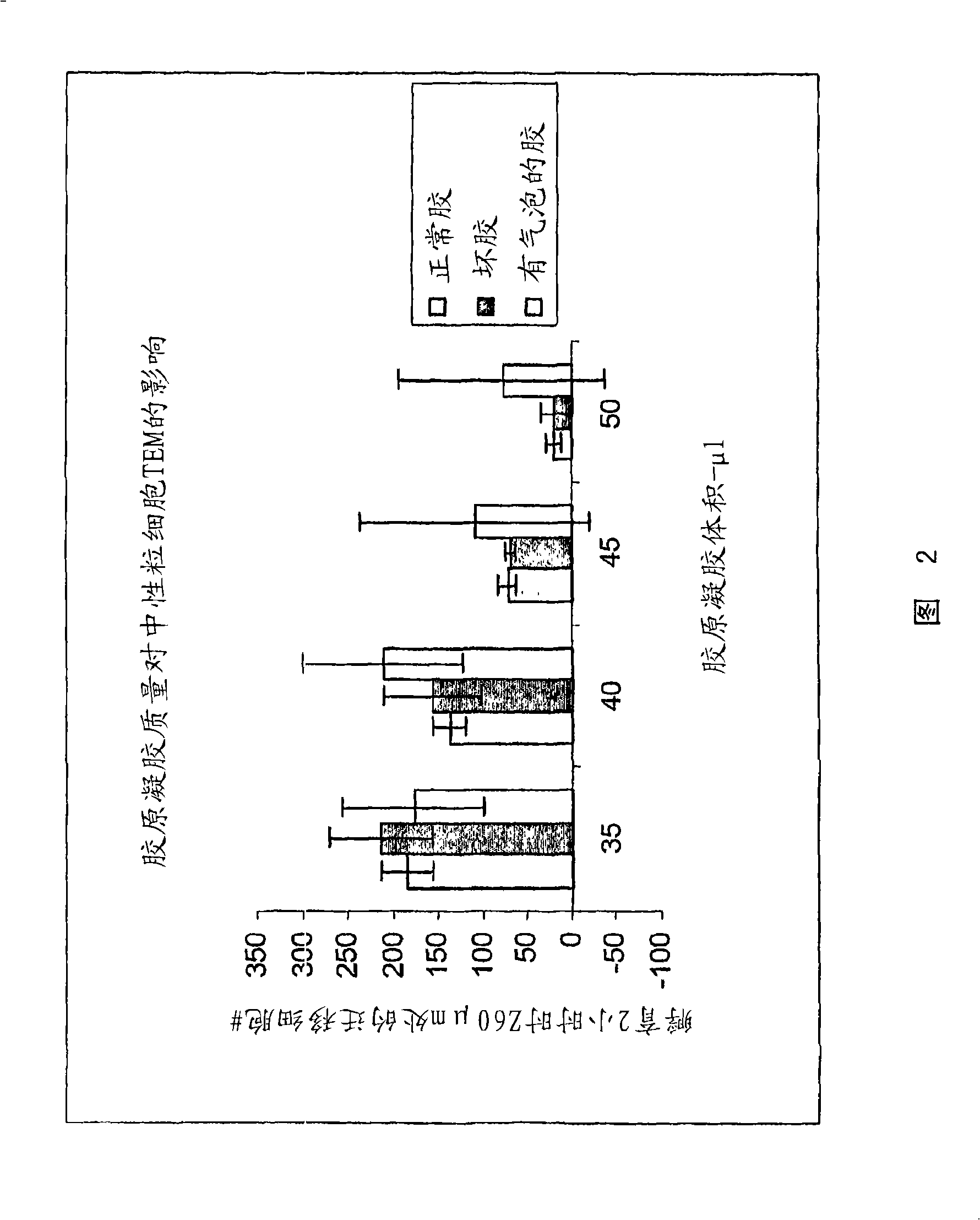
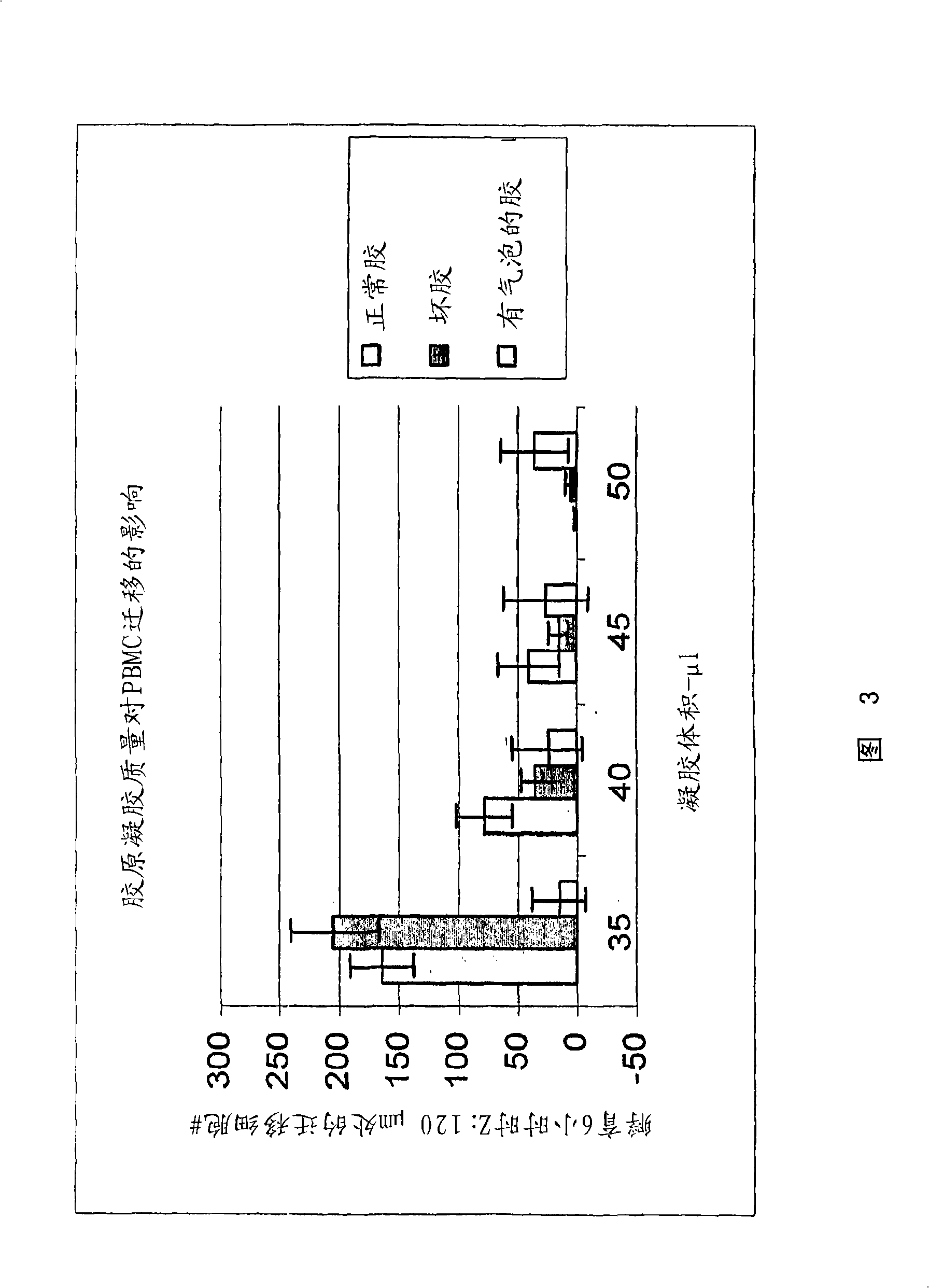
Cell migration assay
The present invention provides compositions and methods for preparation of a three-dimensional transendothelial cell migration (TEM) assay. These compositions and methods are uniquely suited for the high throughput TEM assay, and for the analysis and identification of TEM mediators which inhibit or stimulate this process. The composition for detecting migration of cells comprises a solid layer comprising collagen gel; a first cellular layer in contact with the solid layer and comprising a first cell type; and a second cell type seeded on top of the first cellular layer. Optionally, gelatin is included in the collagen gel. A 96 well plate format is disclosed, the combination with a high throughput cellular scanner enables high throughput TEM assay.
Owner:GE HEALTHCARE BIO SCI CORP
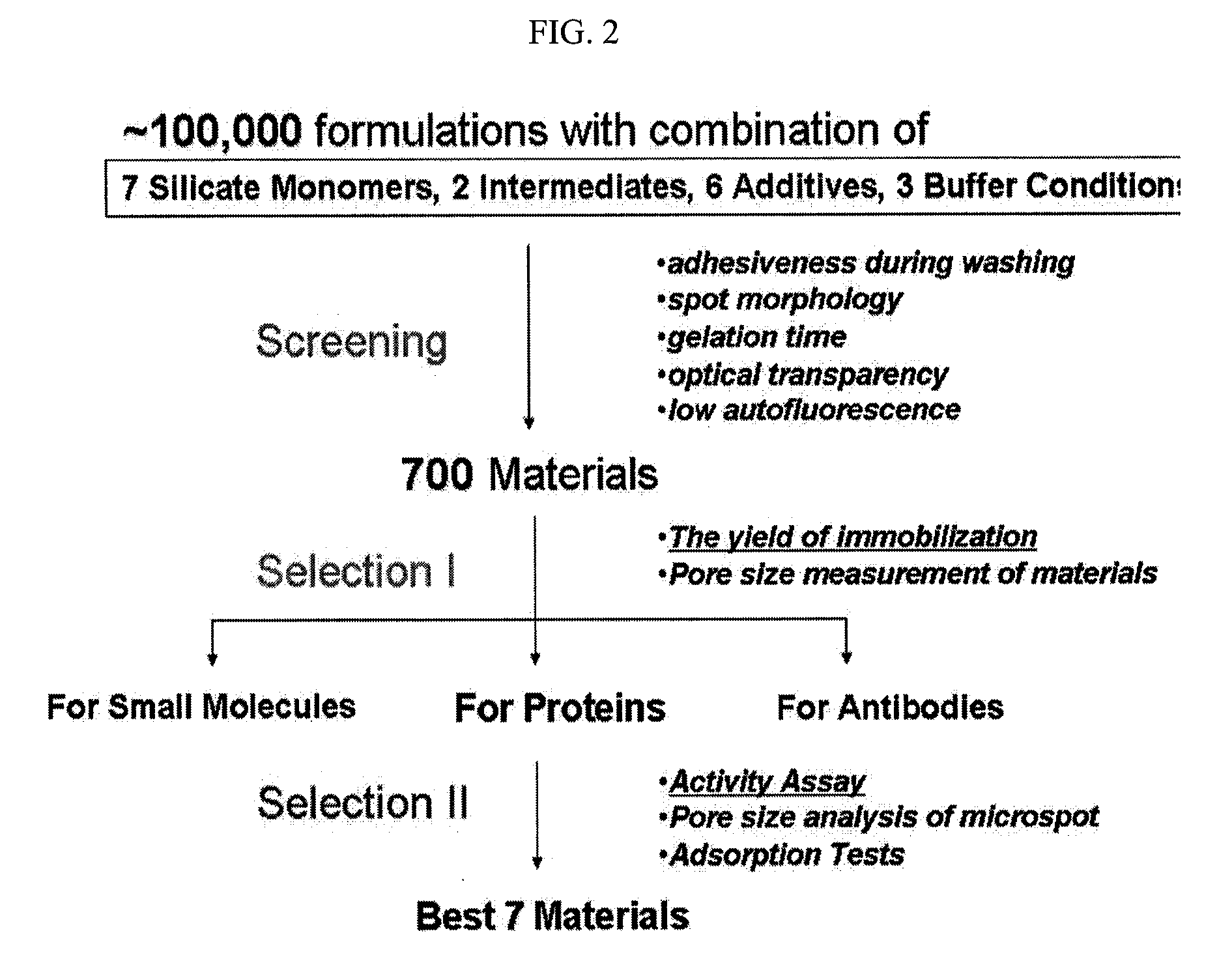
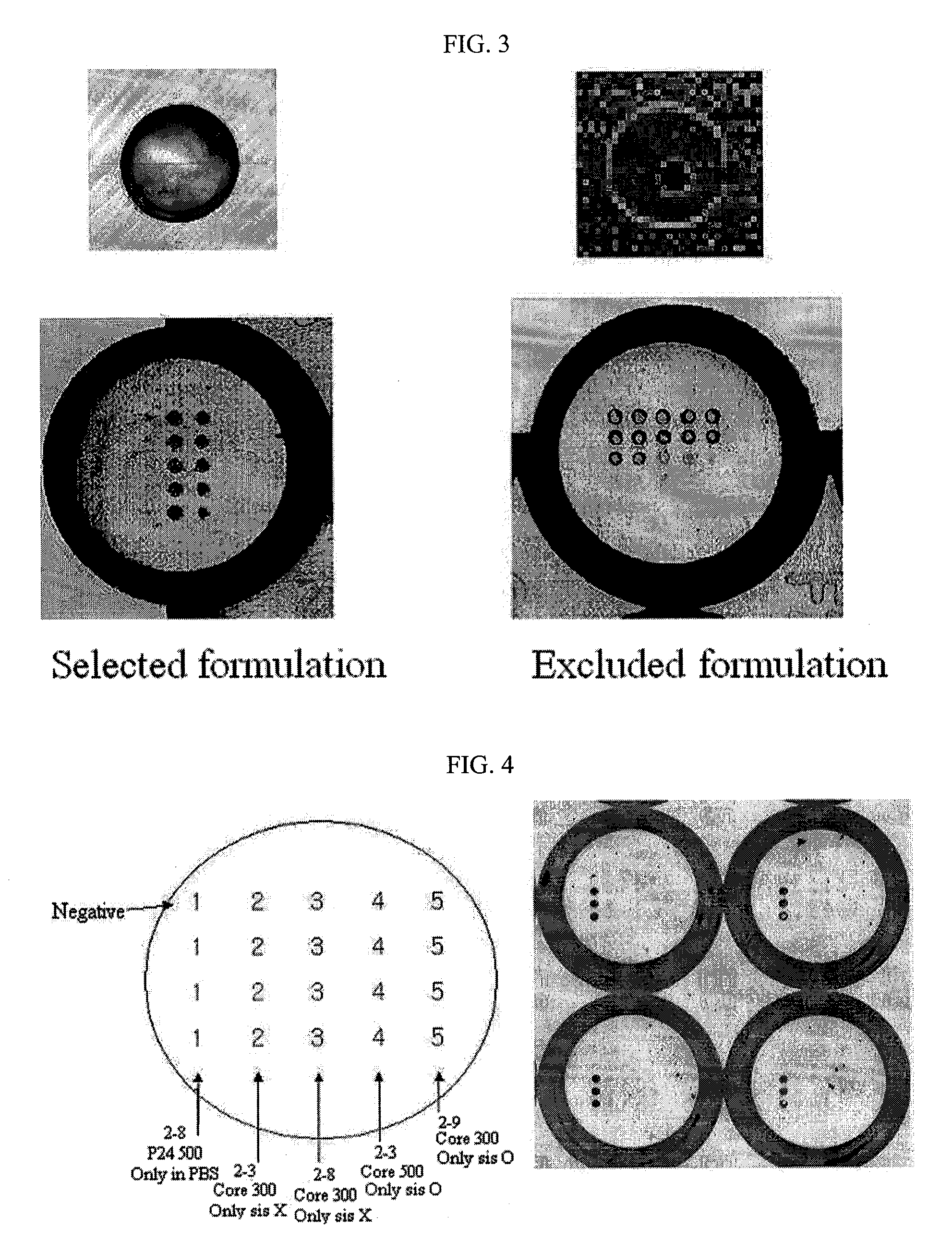
Sol composition for sol-gel biochip to immobilize probe on substrate without surface treatment and method for screening thereof
The present invention relates to a method for screening a sol composition for sol-gel biochips, which is used to immobilize a probe on a surface-untreated substrate, also relates to a sol composition screened by said method and a sol-gel biochip comprising said sol composition immobilized thereon. The sol composition screened by the disclosed method can be mixed with a probe, and the sol mixture can be integrated on 96-well plates, which are widely used in existing bioassays, without surface treatment. Also, the biochip can provide a sensitive and specific good analysis results because this immobilization methods of probe maintain the nature of probes without modification.
Owner:DONGGUK UNIV IND ACADEMIC COOP
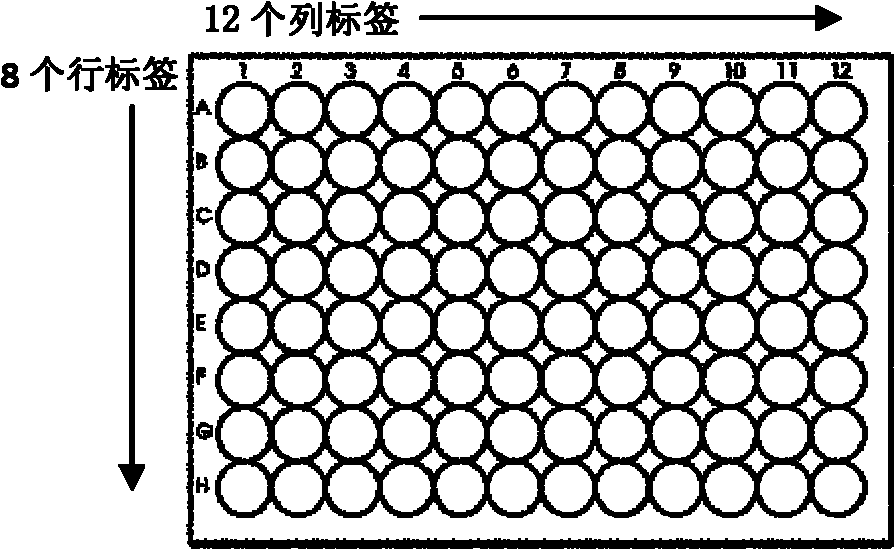
Biochip compatible with 96 pore plate operating system
InactiveCN1390953ASave human effortImprove accuracyMicrobiological testing/measurementMaterial analysisBiotinSolid substrate
A biochip compatible with the automatic operating system of 96-hole enzyme-linked board is composed of solid substrate and perforated frame structure. Said solid substrate is a glass plate, which has been aldehydized or ammonified and treated by biotin and chain avidin for binding biologic moleculae or biologic tissue blocks. Said perforated frame has the sizes same as those of 96-hole enzyme-linked board.
Owner:GENETECH BIOTECH SHANGHAI
Screening method of high flux 96 orifice plate for herbicide
InactiveCN101126712AReduce usageSimple and fast operationMicrobiological testing/measurementColor/spectral properties measurementsContinuous lightScreening method
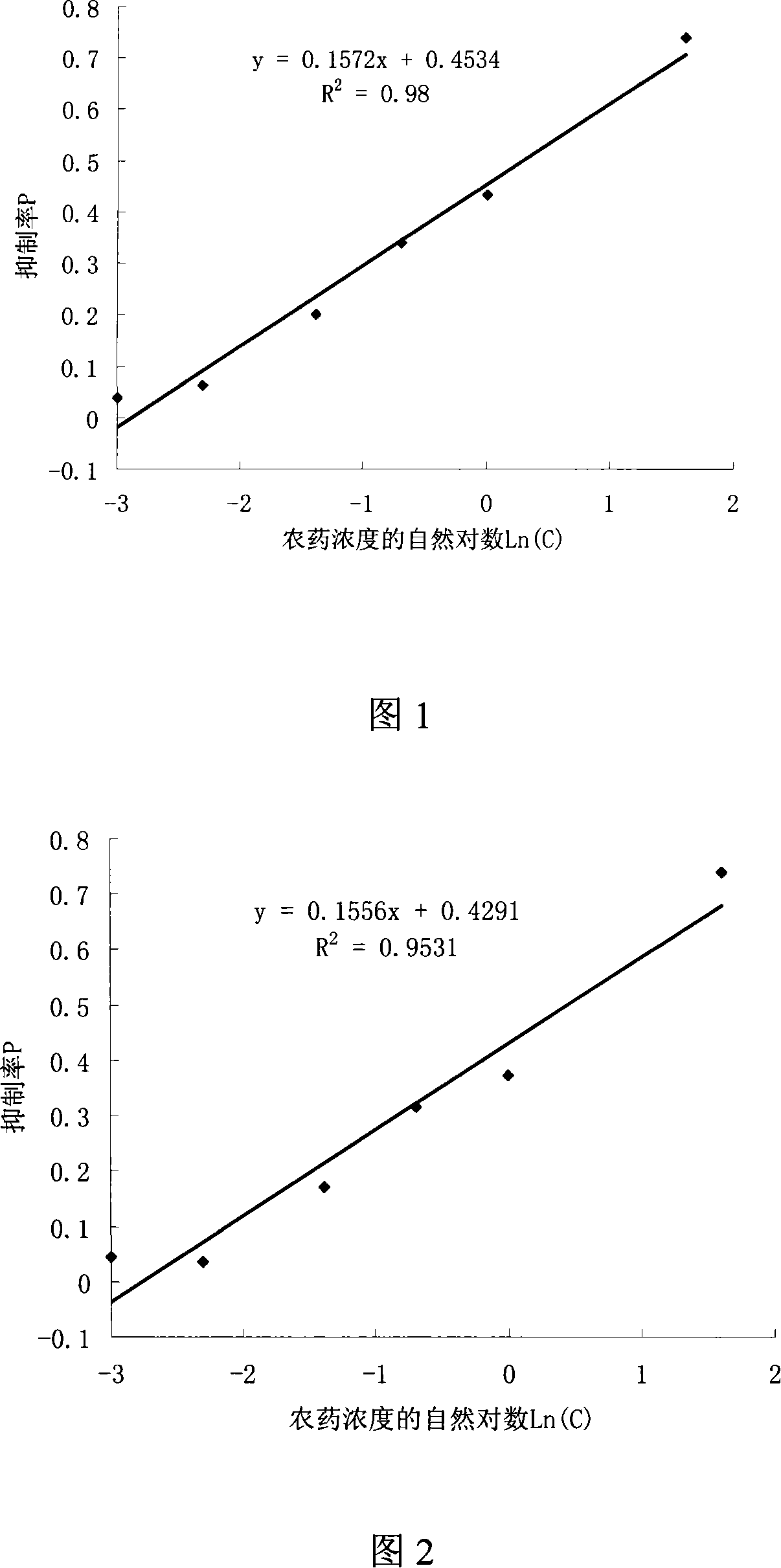
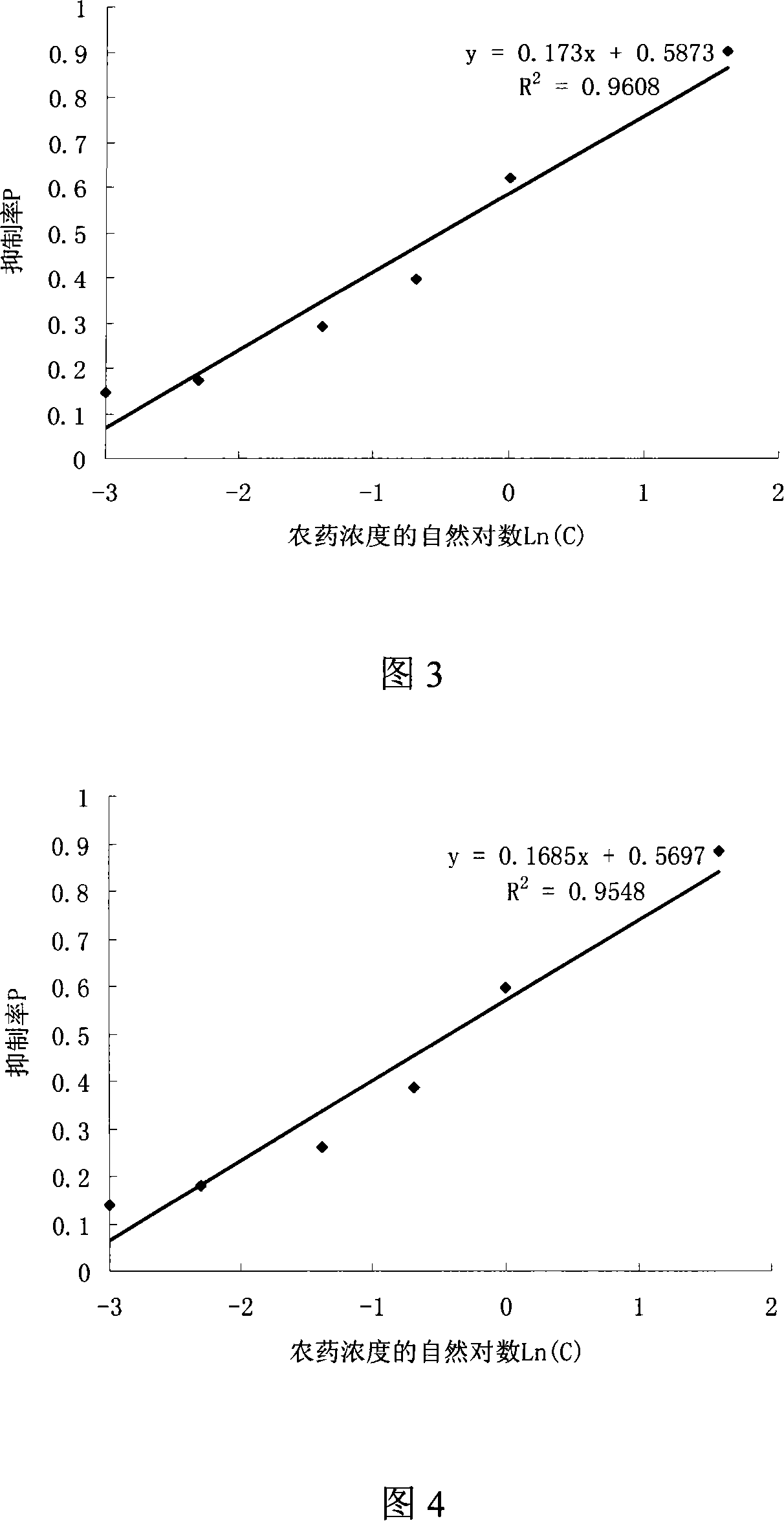
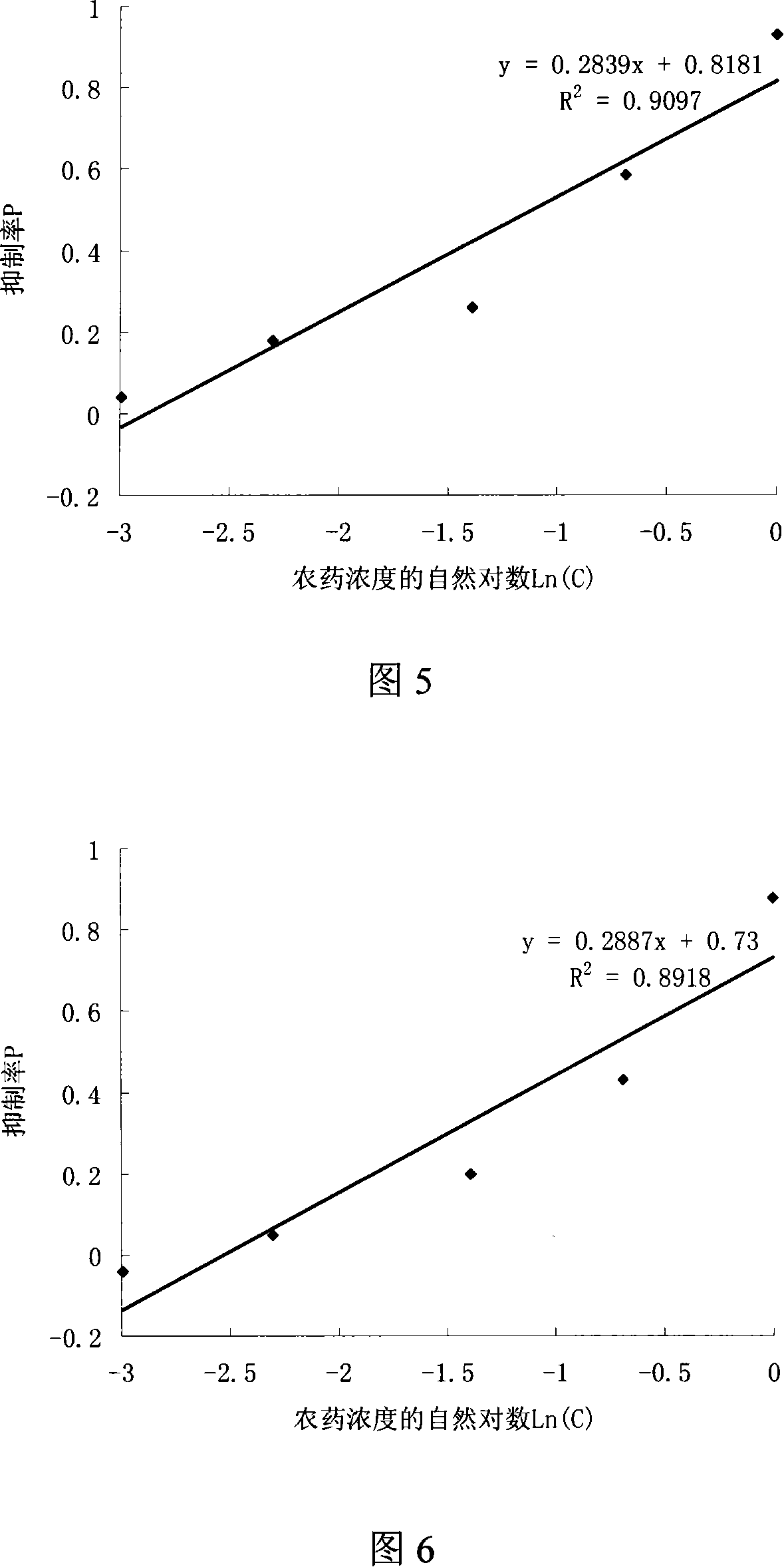
The utility model provides a screening method used for herbicide high flux 96-hole orifice plate. The utility model adopts the technical proposal that: firstly, exponential phase chlorella is inoculated on the 96-hole orifice with a culture medium which is suitable for the chlorella, and the initial inoculating number of the chlorella is 7 to 8 x 105 per mL; secondly, the concentration gradient herbicide is dipped into each hole, and each concentration is provided with two to five duplicate samples as well as an empty control sample. The culturing condition is that: temperature is 25 plus or minus 0.2 DEG C, light is 2000Lx, continuous light is kept, fresh-keeping film is used to seal, no nutrient solution is added, chlorella solution is aerated four to five times every day, the chlorella is cultivated 72 to 144 hours in total, test sample is measured at 630nm absorbance every day, and then the EC50 (median effect concentration) of the herbicide on the chlorella is calculated according to the relationship between the absorbance and the herbicide concentration. Compared with the flask method, the utility model has the advantages of reducing herbicide amount in screening, and having easy operation, fast speed and high sensitivity.
Owner:ZHEJIANG UNIV OF TECH
High throughput screening method of alcohol dehydrogenase
InactiveCN106047985ARapid determinationLow background noiseMicrobiological testing/measurementHigh-Throughput Screening MethodsScreening method
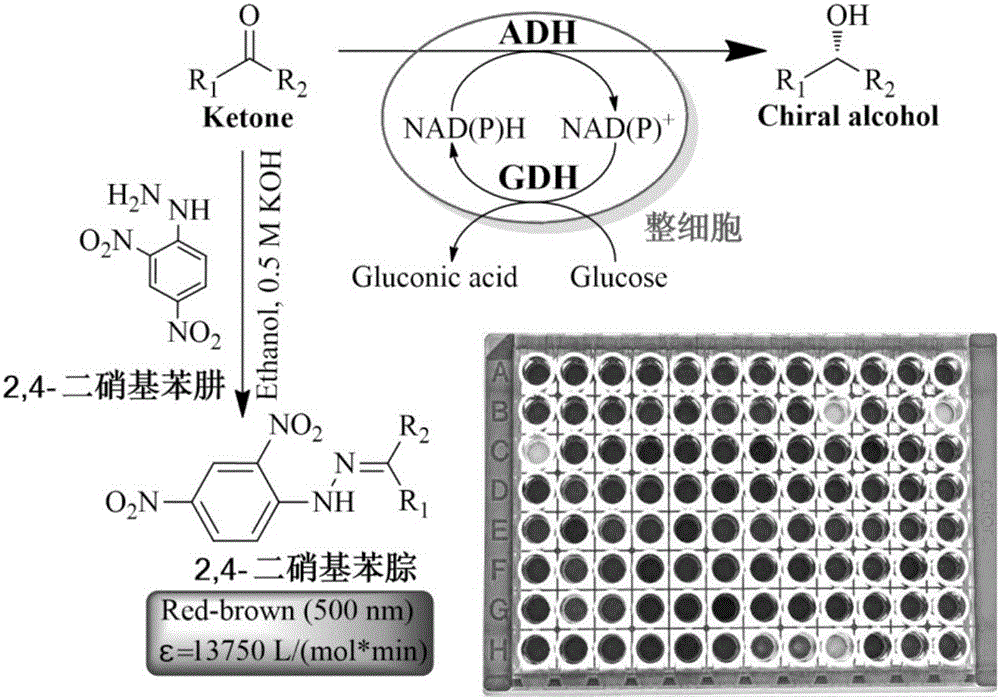
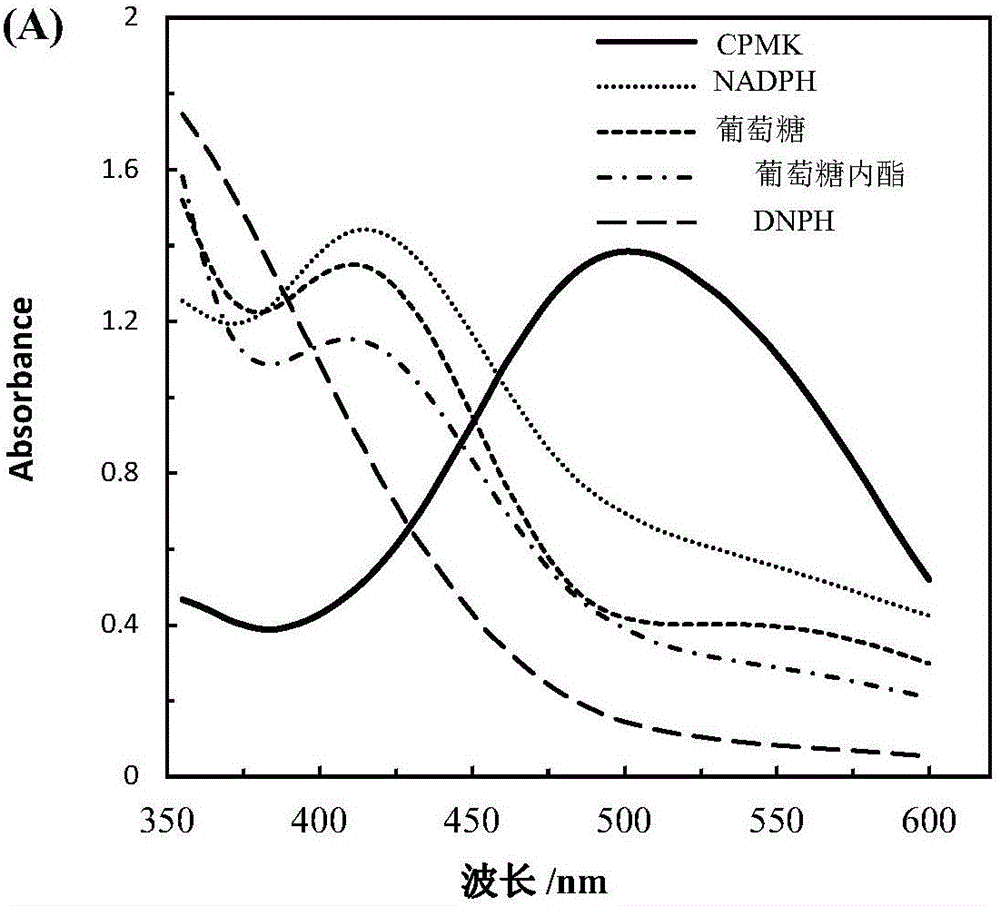
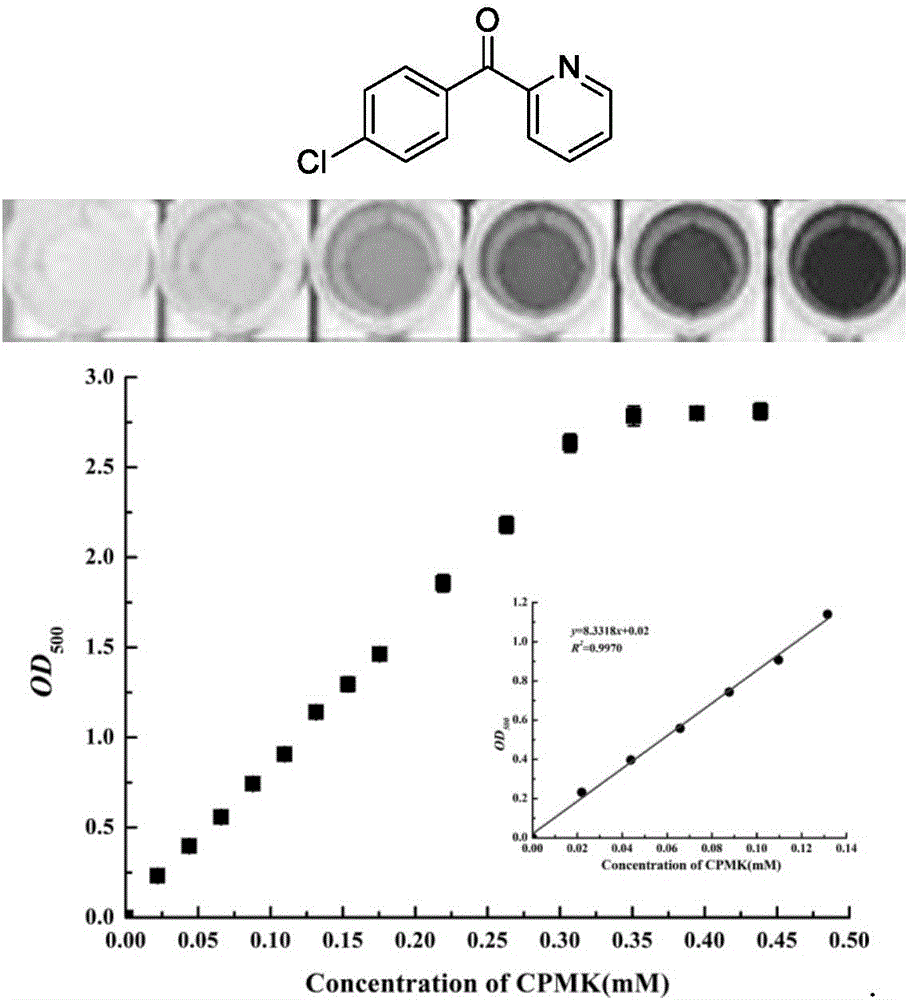
The invention relates to a high throughput screening method of alcohol dehydrogenase, and provides a high throughput screening method for aliphatic series or aryl-substituted ketone alcohol dehydrogenase in 96-well plates. According to the method, aliphatic series or aryl substituted ketone is used for performing a chromogenic reaction with 2,4-dinitrophenylhydrazine under alkaline conditions. A deep-hole plate is used for performing micromation cell culture, no cell wall breaking processing needs to be performed, a traditional screening method of detecting additionally-added expensive coenzyme NAD(P)H absorbance changes is broken through, and the method has the advantages of being low in cost, easy and convenient to operate, sensitive in reaction, accurate in detection, low in equipment requirement and high in universality, and the activity and reaction conversion of alcohol dehydrogenase in a whole-cell sample can be rapidly detected.
Owner:JIANGNAN UNIV
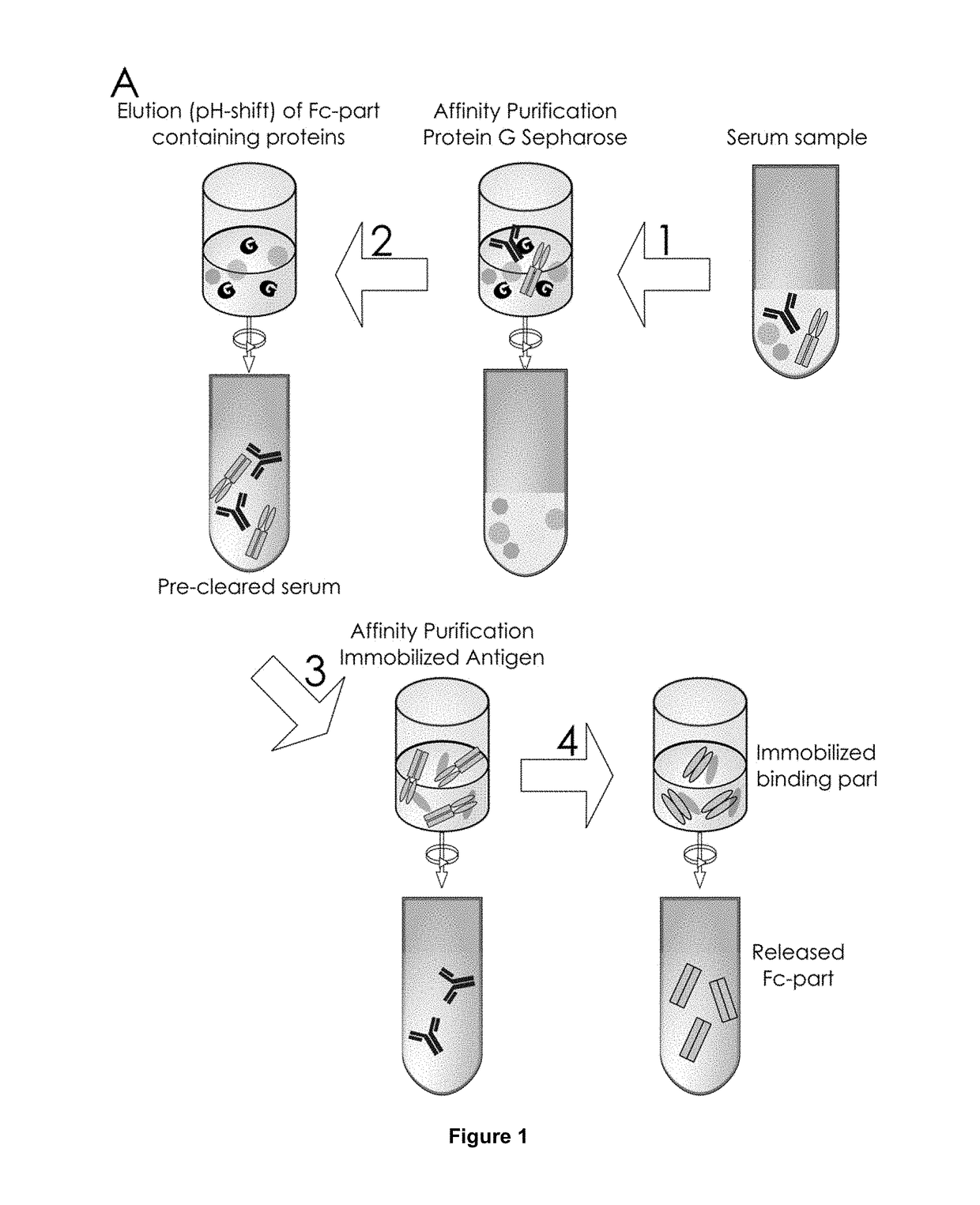
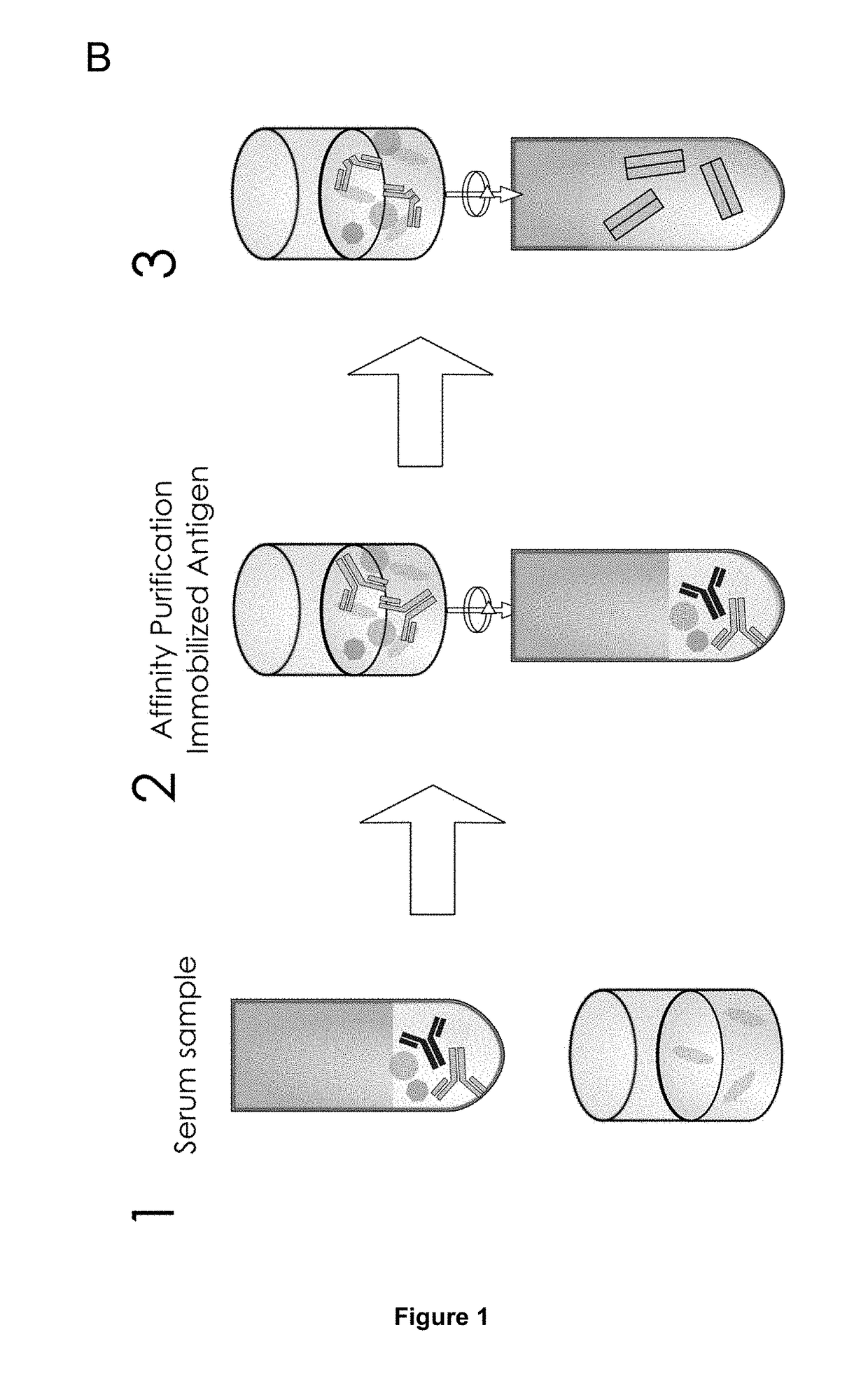
Method of Mapping Glycans of Glycoproteins in Serum Samples
The present invention relates to a method for analyzing glycans of a recombinant glycoprotein in a liquid sample of a mammal. Specifically the method comprises a step of affinity purifying the recombinant glycoprotein from the sample, enzymatically releasing a glycan containing fragment from the immobilized glycoprotein, adding a reference standard containing isotopically labeled glycans, fluorescently label the glycans and analyzing the glycans using LC-MS. The present invention also relates to a method further comprising analyzing the glycans of the immobilized recombinant glycoprotein fragment, further comprising a pre-clearing step of the liquid sample, and releasing the glycans from the immobilized recombinant glycoprotein fragment. The methods allow for the use of a small sample volume and the possibility to operate with high throughput, such as in a 96-well plate sample preparation and are therefore suited to measure pharmacodynamics parameters of a recombinant glycoprotein in a mammal in clinical or pre-clinical studies.
Owner:HEXAL AG
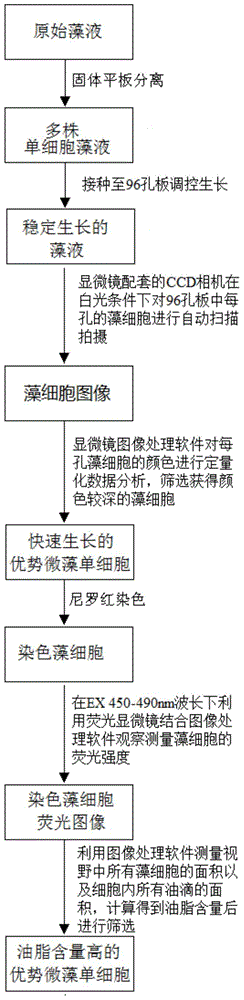
Method for screening microalgae unicells which grow fast and are high in grease content through fluorescence microscope
ActiveCN104568881AImprove work efficiencyReduce labor time costsFluorescence/phosphorescenceFluorescence microscopeCell separation
The invention relates to a biomass energy utilization technology, and aims to provide a method for screening microalgae unicells which grow fast and are high in grease content through a fluorescence microscope. The method for screening the microalgae unicells which grow fast and are high in grease content through the fluorescence microscope includes the following steps: taking a microalgae liquid for unicell separation; enlarging the cultivation to obtain a liquid containing a plurality of unicellular microalgae strains; then inoculating the unicellular microalgae liquid to 96-mesh plates for cultivation until the stable phase; screening the superior microalgae unicells with a high growth rate and dying using Nile red fluorochrome / DMSO; calculating the grease content of the microalgae unicells, so as to screen the superior microalgae unicells with a high grease content. According to the invention, superior algal strains which grow fast and are high grease content can be quickly screened from the algal cells on the 96-well plates in about 10 minutes, which greatly improves the working efficiency of screening target algal strains and reduces labor time and cost.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com