Chemiluminescence detection kit of swine foot-and-mouth disease 3ABC and 2C antibodies
A technique for chemiluminescent detection and porcine foot-and-mouth disease, applied in the field of immunological detection, to prevent uneven coating, improve quality, and reduce ionization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
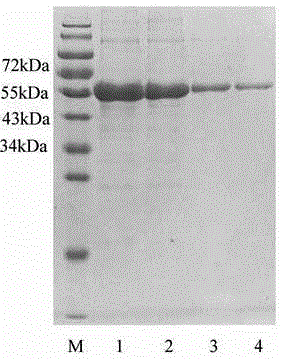
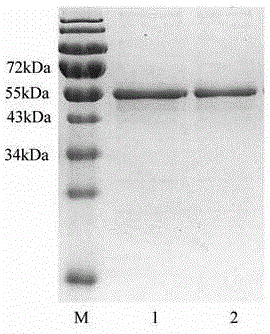
[0030] Example 1 Preparation of 3ABC and 2C fusion protein
[0031] Total RNA was extracted from the vesicle fluid of pigs infected with foot-and-mouth disease virus according to the operating instructions of the RNeasy® Mini Kit kit; using the extracted RNA as a template, cDNA was synthesized using SMART® MMLV reverse transcriptase and oligo dT primers (purchased from Takara); the design Primers, using the synthesized cDNA as a template, first use primers 3ABC-F and 3C-46-R to amplify the first gene, then use 3C-46-F and 3C-163-R to amplify the second gene, and then The third gene was amplified with 3C-163-F and 3ABC-R. Finally, the three amplified genes are fused into a mutated full-length 3ABC gene by fusion PCR, wherein the sequence of the 3ABC gene is shown in SEQ ID NO:1. Using 2C-F and 2C-R as primers, the full-length 2C gene was amplified according to standard PCR operations, wherein the 2C gene sequence is shown in SEQ ID NO:3. The primer sequences are shown in Tabl...
Embodiment 2
[0036] Example 2 Optimization and establishment of the CLIA method for detecting porcine foot-and-mouth disease 3ABC and 2C antibodies
[0037] The pig serum with high infection titer 60 days after FMDV infection was used as the standard positive control serum, and the blocking rate of the serum was 98% when detected by the FMDVPrioCHECK® NSP ELISA kit; the healthy pig serum that had not been immunized with any vaccine was used as the standard negative control. Determine the optimal 3ABC antigen coating concentration and serum dilution by means of checkerboard titration. Among them, the 3ABC protein was made in gradients of 2ug, 1ug, 500ng and 250ng, and the standard negative and positive serum was made 10, 20, 40 and 80 times respectively. dilution. By comprehensively considering the signal-to-noise ratio and economic factors, the protein coating condition was mixed coating 250ng / mL 2C protein and 250ng / mL 3ABC protein antigen, and the serum dilution was set at 1:40.
Embodiment 3
[0038] Example 3 Sensitivity and specificity evaluation
[0039] 1. Establishment of serum plate
[0040] a. A total of 63 serum samples came from clinically healthy pigs. These pigs had not been vaccinated. They were tested with the liquid-phase blocking ELISA kit for foot-and-mouth disease developed by Lanzhou Veterinary Research Institute. These sera had no corresponding O, A, and Asia1 antibodies . These sera were used to evaluate the diagnostic specificity of a CLIA kit co-coated with 3ABC and 2C (3ABC / 2C CLIA kit).
[0041] b. A total of 532 serum samples were collected from pigs that were clinically healthy and immunized with inactivated foot-and-mouth disease vaccine, and these serum samples were collected 7-30 days after immunization. These sera were used to evaluate the diagnostic specificity of the 3ABC / 2C CLIA kit.
[0042] c. A total of 117 serum samples were from experimentally challenged pigs. These serum samples are serum samples collected 10 days after the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com