Neurotrophin-derived peptide sequences
一种神经营养因子、肽序列的技术,应用在神经营养因子衍生的肽序列领域
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0216] The preparation of polyclonal antibodies is well known to those skilled in the art. See, for example, Green et al., 1992, Production of Polyclonal Antisera, in Immunochemical Protocols (Manson ed.), pp. 1-5 (Humana Press); Coligan et al., Production of Polyclonal Antisera in Rabbits, Rats, Mice and Hamsters, in Current Protocols in Immunology , Section 2.4.1, which are incorporated herein by reference.
[0217] The preparation of monoclonal antibodies is likewise routine. See, eg, Kohler and Milstein, Nature, 256:495-7 (1975); Coligan et al., Sections 2.5.1-2.6.7; and Harlow et al., Antibodies: A Laboratory Manual, page 726, Cold Spring Harbor Pub. (1988) . Monoclonal antibodies can be isolated and purified from hybridoma cultures by a variety of well-established techniques. These separation techniques include affinity chromatography using Protein A-Sepharose, size exclusion chromatography, and ion exchange chromatography. See eg Coligan et al., Sections 2.7.1-2.7.1...
Embodiment 1
[0286] Example 1: Stimulation of neurite outgrowth
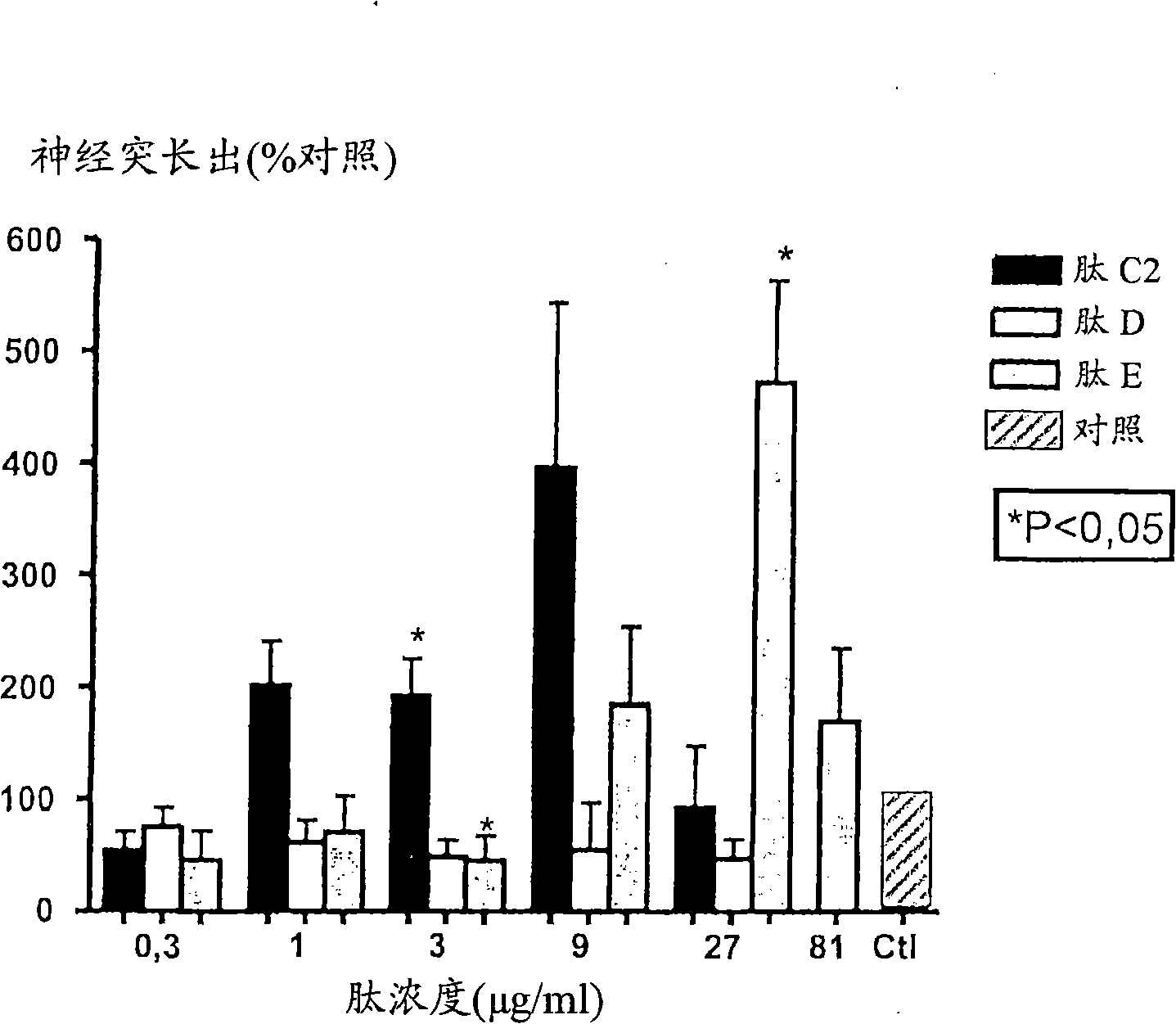
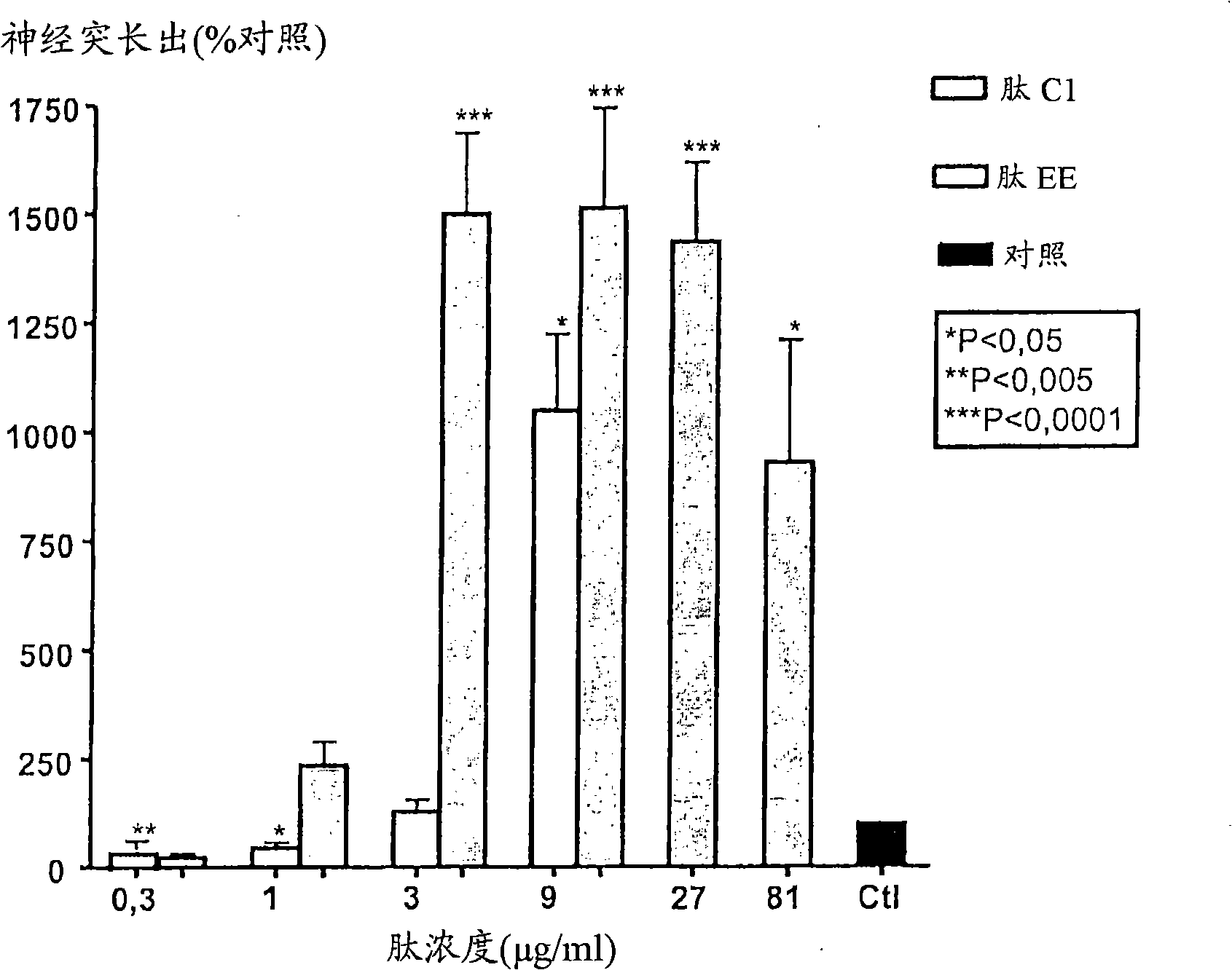
[0287] CGN prepared as above were treated with peptides NGF-C2, NGF-D, NGF-E, NGF-C1, NGF-EE, BDNF-E or BDNF-EE at different concentrations (3-81 μg / ml).
[0288] From figure 1 , 2 , 4 and 5, it can be seen that the peptides NGF-C2 (motif II), NGF-E (motif III), NGF-C1 (motif I), NGF-EE (motif III) containing the specified structural motifs , BDNF-E (motif III) or BDNF-EE (motif III) all significantly stimulated neurite outgrowth from primary neurons in culture. In contrast, the peptide NGF-D, which lacks the essential hydrophobic residues of motif I, does not have neuritic activity ( figure 1 ).
Embodiment 2
[0289] Example 2: Stimulation of neuronal cell survival
[0290] The effect of different peptides on neuronal survival was examined both in vivo and in vitro.
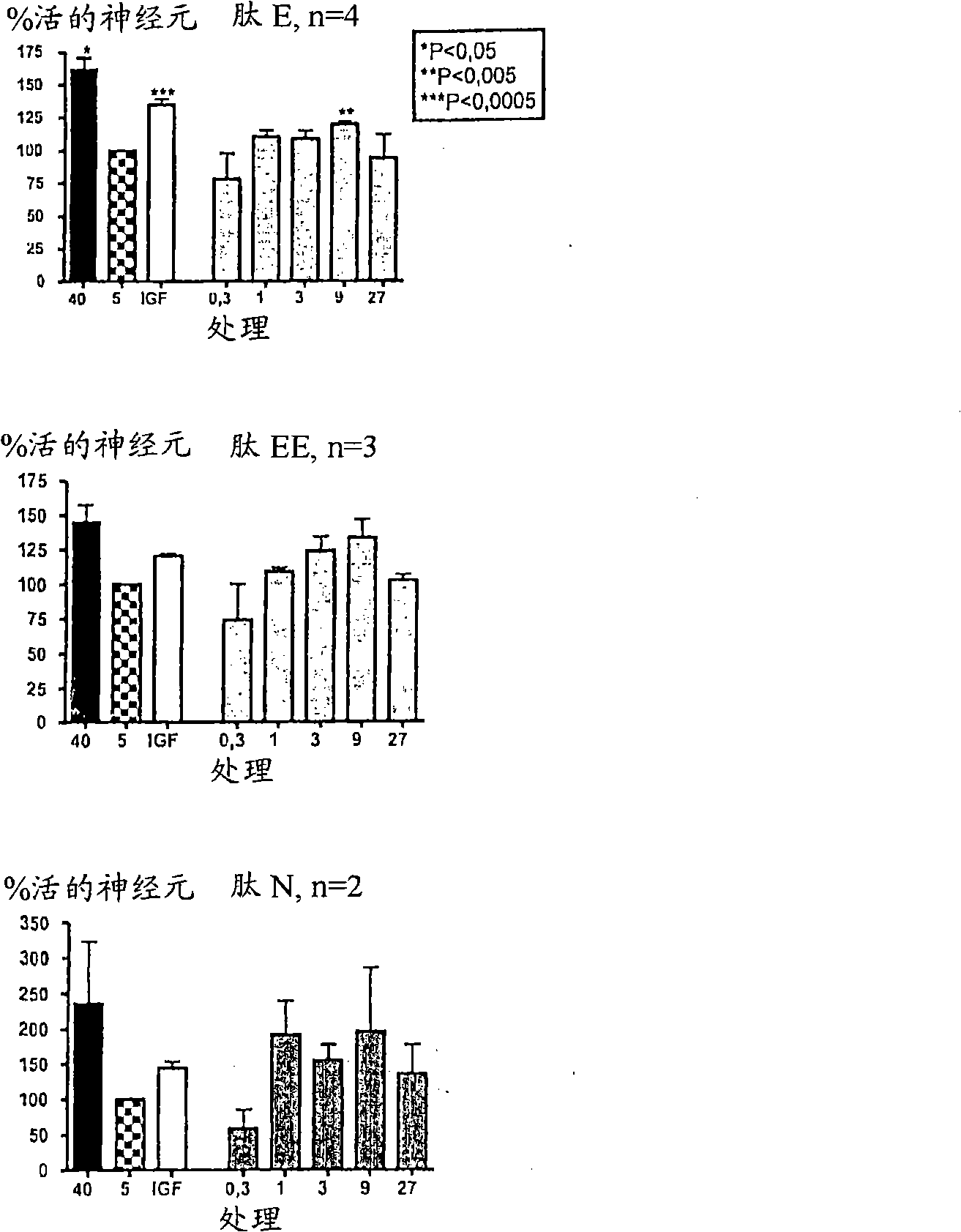
[0291] For in vitro experiments, cultures of CGR neurons were prepared as above and treated with different concentrations of NGF-E, NGF-EE or NGF-N.
[0292] From image 3 As can be seen, all tested peptides significantly promote the survival of CGN in culture.
[0293]In vivo test - Focal brain injury of the right parietofrontal cortex by applying a piece of dry ice (-78°C) directly to the skull of a mouse for 30 seconds and to a rat skull for 60 seconds, as previously described in detail (Penkowa et al., 2003). Focal brain injury of the right fronto-parietal cortex was induced by applying a piece of dry ice (-78°C) directly to the mouse skull for 30 s and to the rat skull for 60 s, as previously described. described in detail (Penkowa et al., 2003). Following treatment, sections were incubated overnight at 5°C w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com