Novel low dose pharmaceutical compositions comprising nimesulide, preparation and use thereof
A low-dose, solvate technology, applied in the field of preparation of this dosage form, can solve the problems of poor water solubility and wettability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
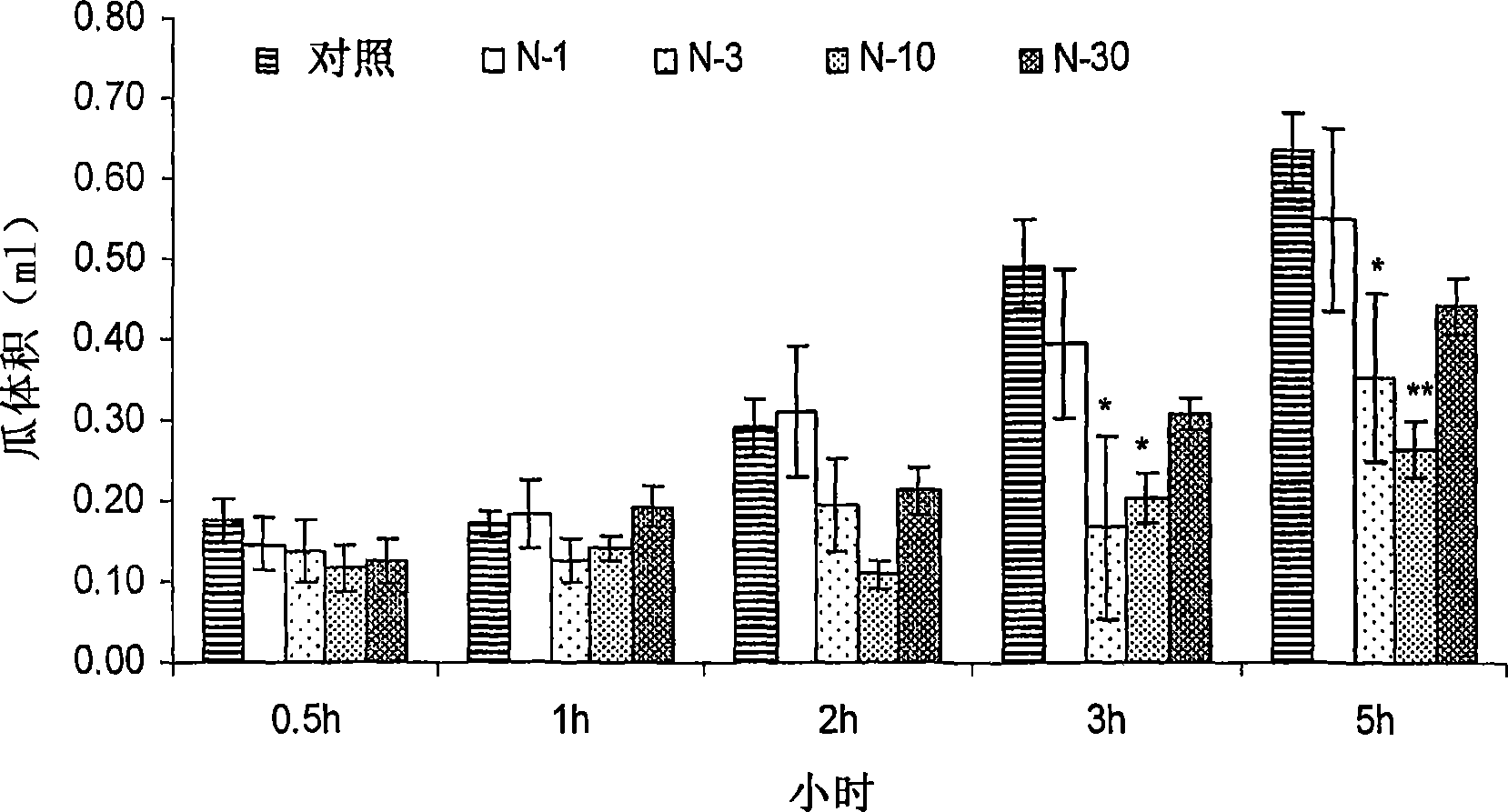
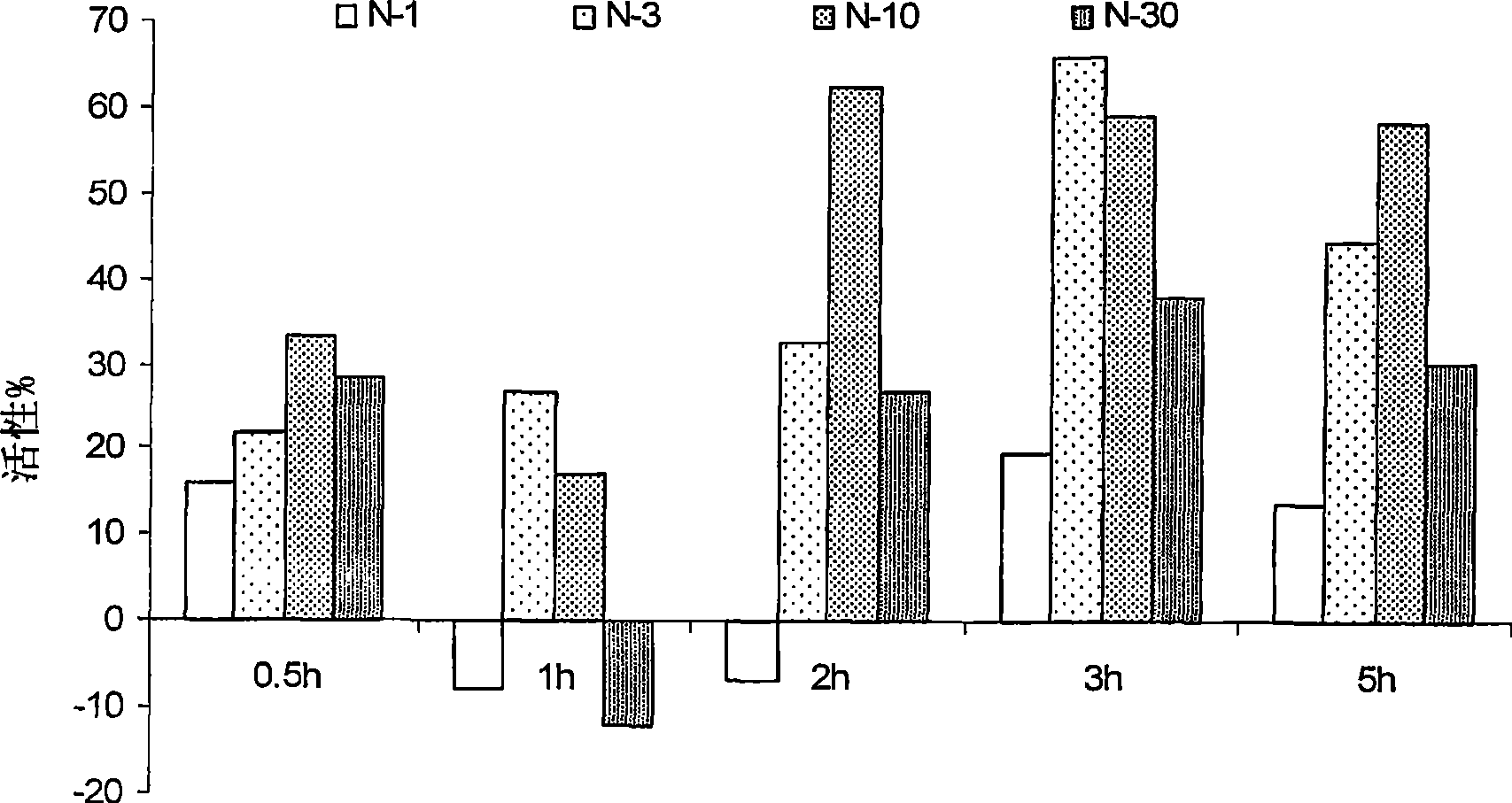
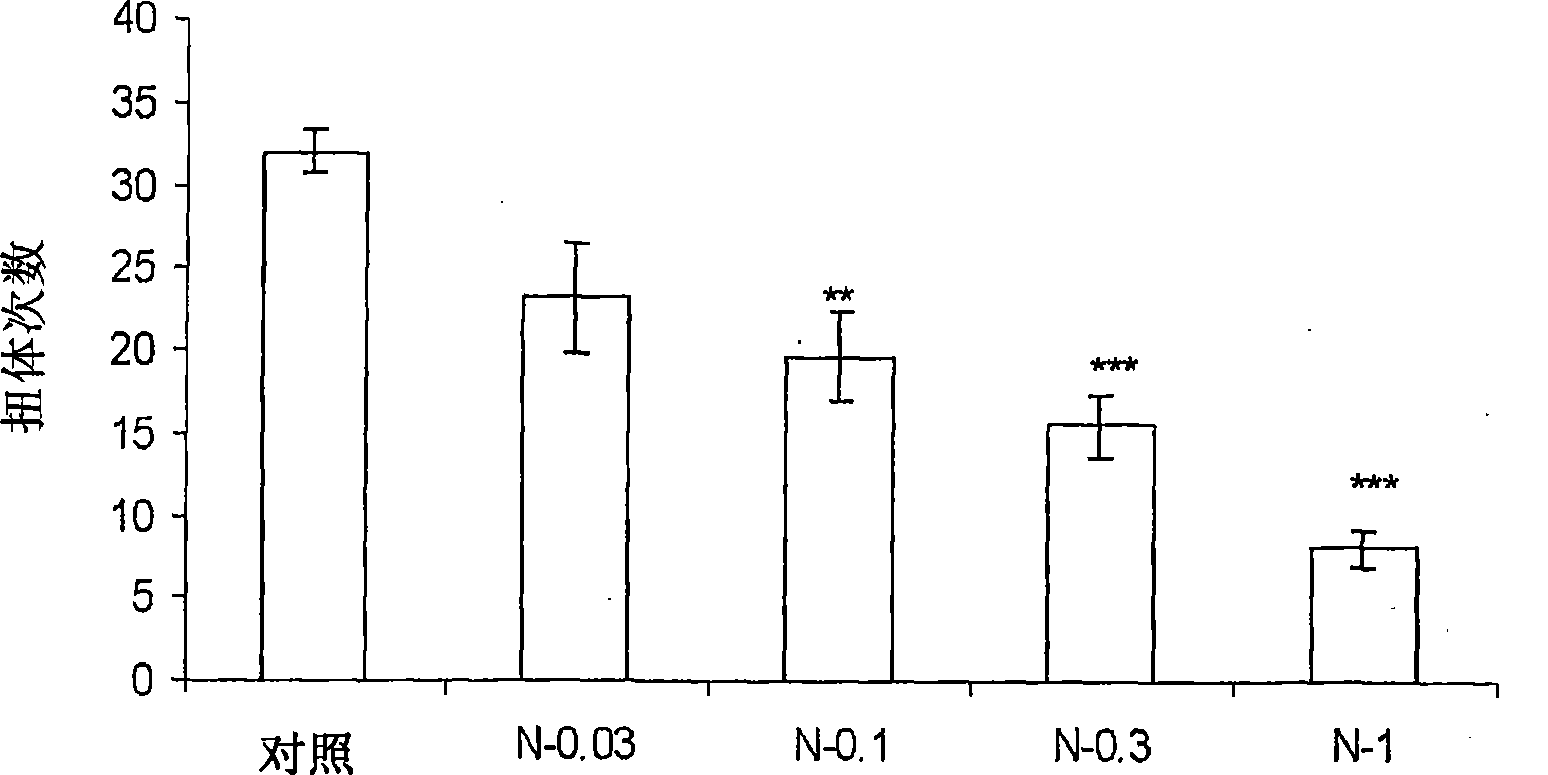
[0022] Nimesulide is routinely administered as 100-200 mg tablets or capsules twice a day, or as a 50 mg / ml suspension for the treatment of inflammatory conditions, pain, arthritis and the like. High dose compositions of nimesulide are associated with dose-related side effects such as gastric or hepatotoxicity, even some heart diseases or any other condition due to enzymatic inhibition of cyclooxygenase. The inventors attempted to reduce or at least alleviate the dose-related side effects associated with nimesulide by reducing the normally administered dose of nimesulide. In addition, low dose compositions have improved solubility, thus improved bioavailability, and are easier to formulate. In addition, the novel composition requires a smaller amount of excipients and therefore has a smaller size than conventionally available dosage forms, which in turn leads to better acceptance by patients. Preferably, the compositions of the present invention do not require the use of any ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com