Prolonged improvement of renal function comprising infrequent administration of an AAIRA
A technology for renal function and use, applied in the field of prolonged improvement of renal function including low-frequency administration of AA1RA, which can solve problems such as increased cardiac workload, difficulty in effective treatment, arrhythmia, and cardiovascular system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0116] Example 1: Pharmacokinetic study of KW-3902
[0117] Subjects received a single intravenous dose of 1 mg, 2.5 mg, 5 mg, 10 mg, 20 mg, 30 mg, 40 mg, 50 mg or 60 mg of KW-3902 as indicated. Serum concentrations of KW-3902 and its M1-anti-metabolite were measured using routine analytical methods. Pharmacokinetic values are determined using conventional mathematical formulas. Data are presented in Table 1.
[0118] Table 1
[0119]
[0120]
[0121] T of 30mg dose of KW-3902 1 / 2 The average is 14.7 hours.
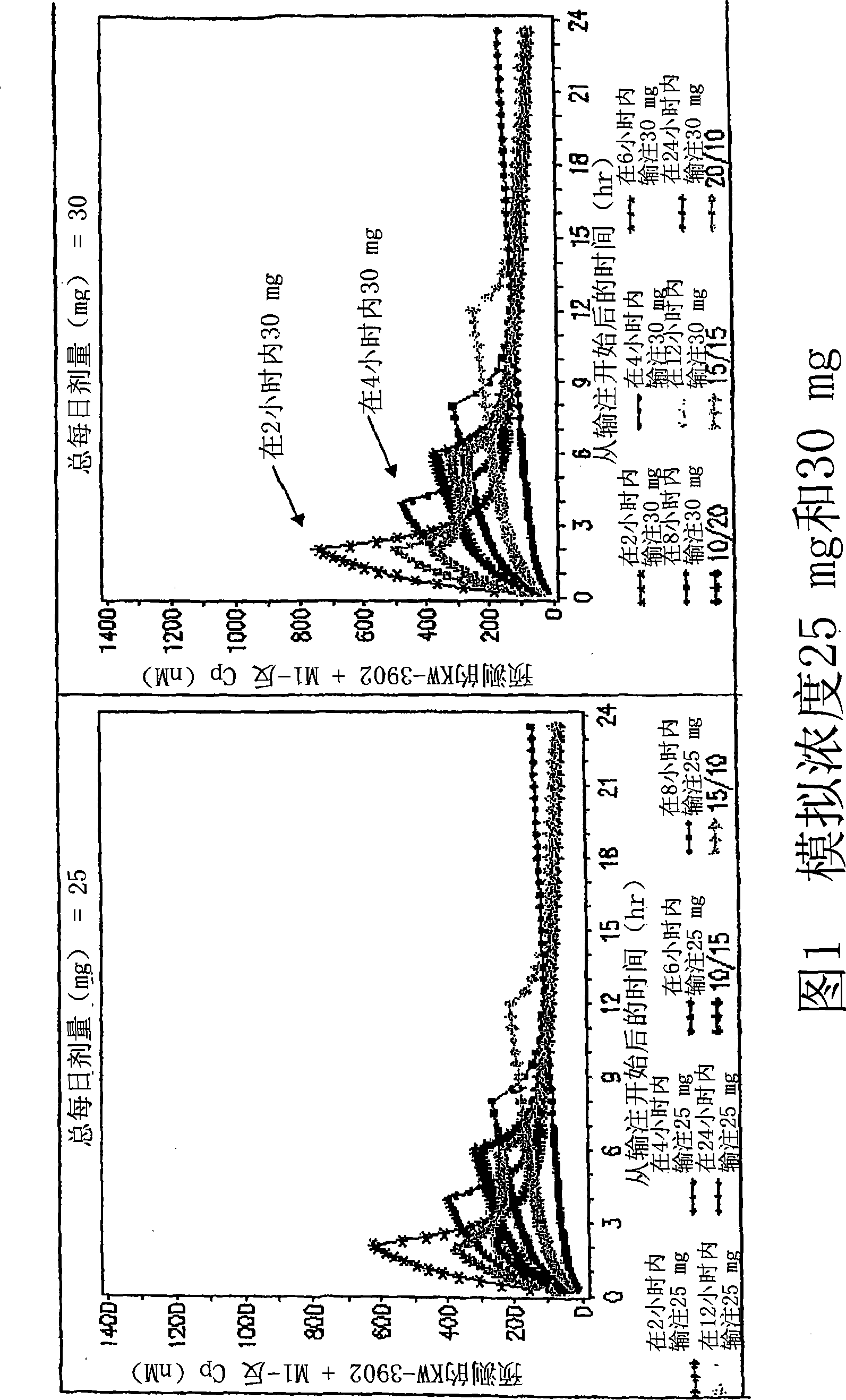
[0122] These data were extrapolated to generate curves showing simulated serum concentrations of KW-3902 and M1-trans following intravenous infusion of KW-3902. Curves for simulated concentrations are shown in Figures 1A and 1B. Briefly, both 1A and 1B demonstrate the predicted serum concentrations of KW-3902 and M1-reverse over time following a single 25 mg or 30 mg intravenous dose of KW-3902. As indicated in the legend, the curves show predicted conce...
example 2
[0124] Example 2: Treatment of Individuals with Stable CHF
[0125] A double-blind, multicentre, cross-over design controlled study was performed as follows: approximately 23 individual outpatients were randomized into groups. Subject has an estimated creatinine clearance between 30 mL / min and 80 mL / min and is taking >80 mg of furosemide daily. Subjects receive at least two treatments separated by at least 3 days, wherein the median interval between treatments is 6 days. In addition to receiving 80 mg intravenous furosemide over 120 minutes, each patient received 30 mg intravenous KW-3902 or placebo over 120 minutes. Patients who received KW-3902 at the first visit received placebo at the second visit and vice versa. Neodiatrizoate (Iothalamate) (CONRAY TM ) and p-aminohippurate infusion, the corresponding assessment of glomerular filtration rate (GFR) and renal plasma flow (RPF). Neodiatrizoate and p-aminohippuprate were administered 180 minutes prior to KW-3902 / placebo...
example 3
[0132] Example 3: KW-3902 improves renal function in CHF patients refractory to standard diuretic therapy Function
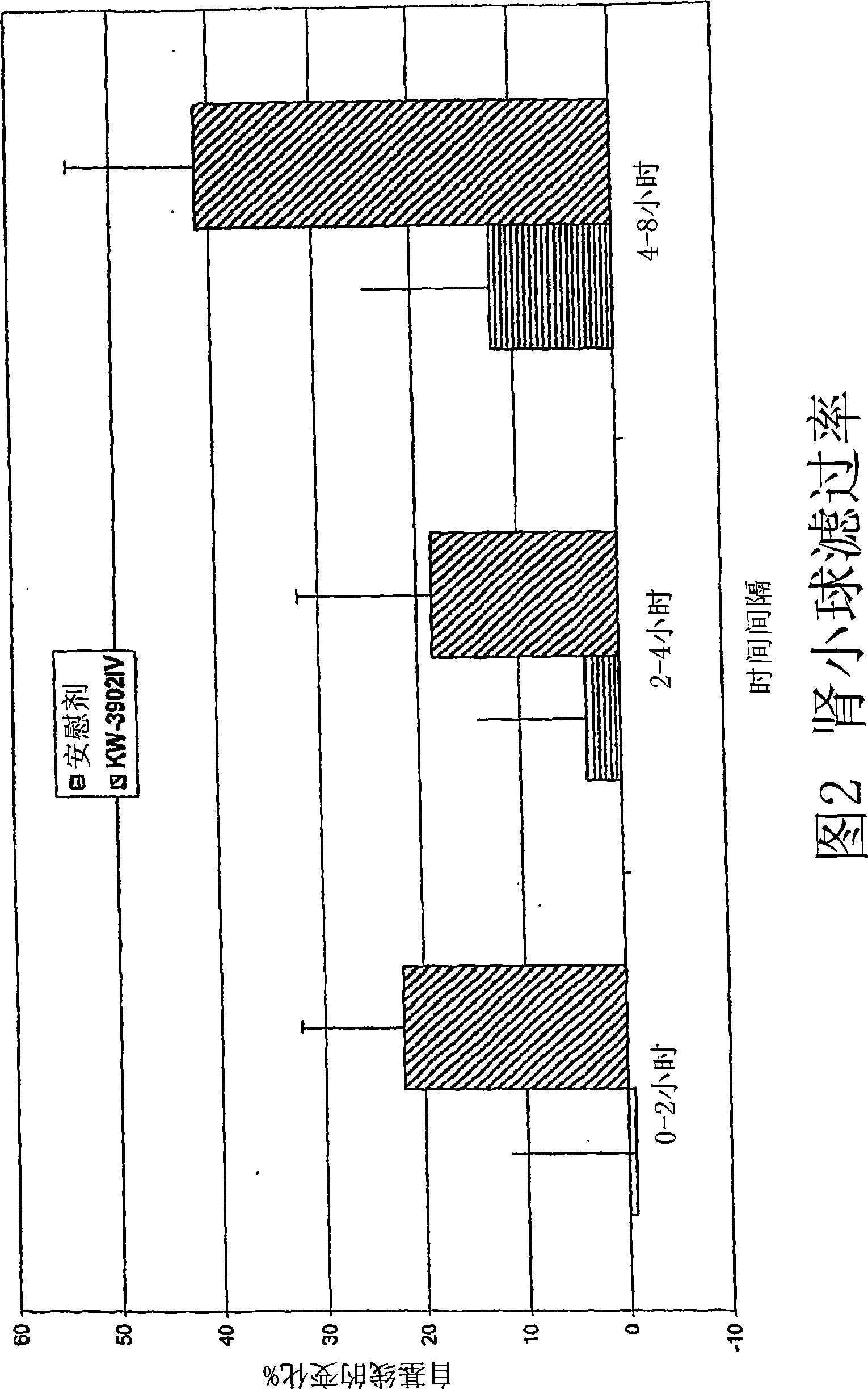
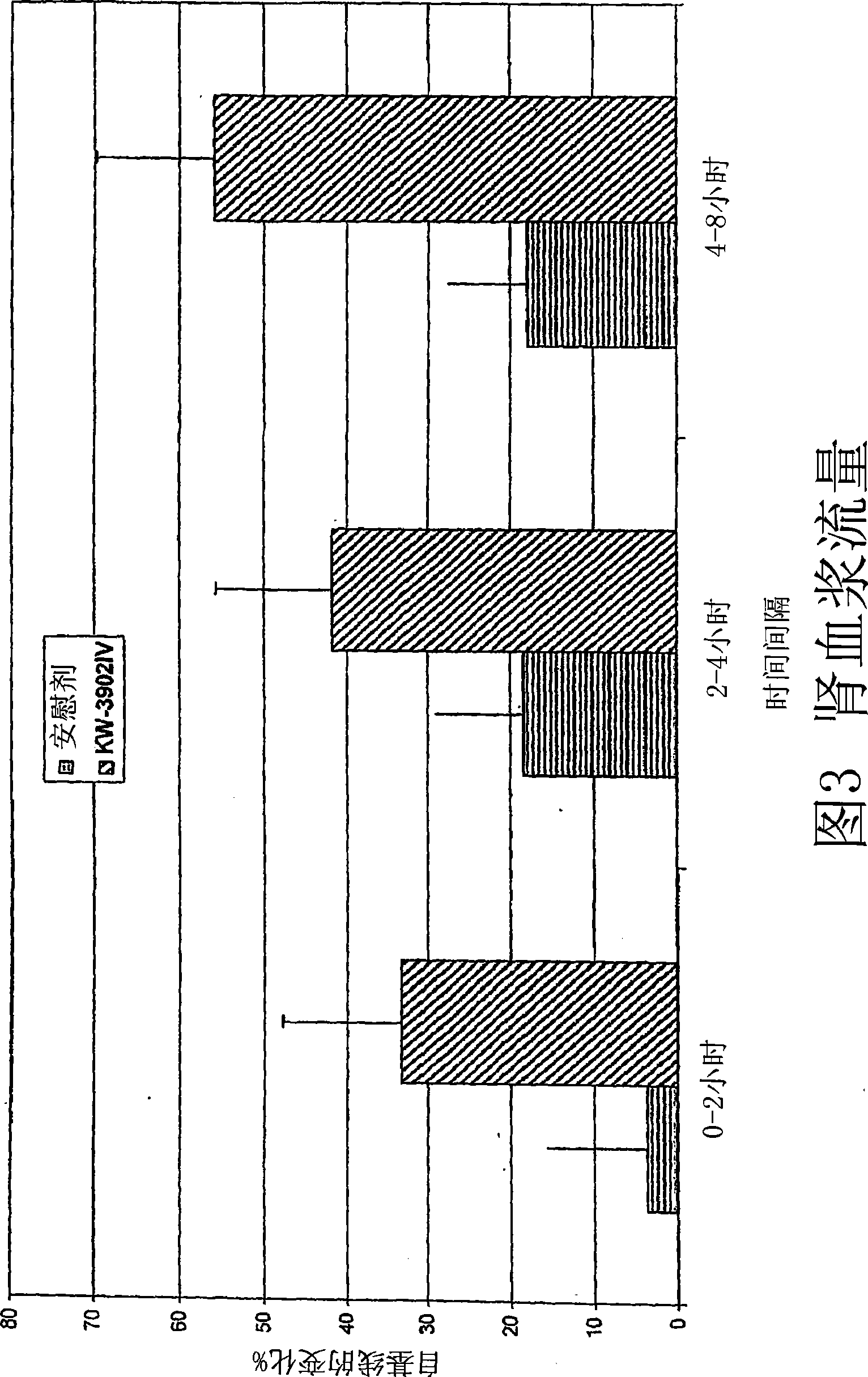
[0133] A double-blind placebo-controlled study was performed as follows: 35 individuals with congestive heart failure refractory to standard diuretic therapy were identified. Subjects were randomized and divided into four treatment groups: (1) placebo (n=11); (2) 10 mg KW-3902 (n=8); (3) 30 mg KW-3902 (n=7); and (4 ) 60 mg KW-3902 (n=7). Discontinue the patient's diuretic therapy at least 5 hours prior to treatment. Beginning at -3 hours, urine was collected for volume and creatinine clearance measurements. At 0 hours, patients were given an intravenous infusion of KW-3902 or placebo along with the patient's current diuretic regimen. Blood and urine samples were collected at 0, 3, 6, 9, 12, and 24 hours after study drug administration. The change in creatinine clearance (mL / min) from baseline at 0-3 hours, 3-6 hours, 6-9 hours, 9-12 hours, and 12-24 hour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com