Pharmaceutical composition containing a tetrahydrofolic acid
A technology of tetrahydrofolate and composition, which is applied in the directions of drug combination, medical preparation containing active ingredients, medical formula and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0178] Example 1 - Direct Compression; Microcrystalline Cellulose
[0179] 80 mg tablet cores with the following composition are prepared by direct compression:
[0180]
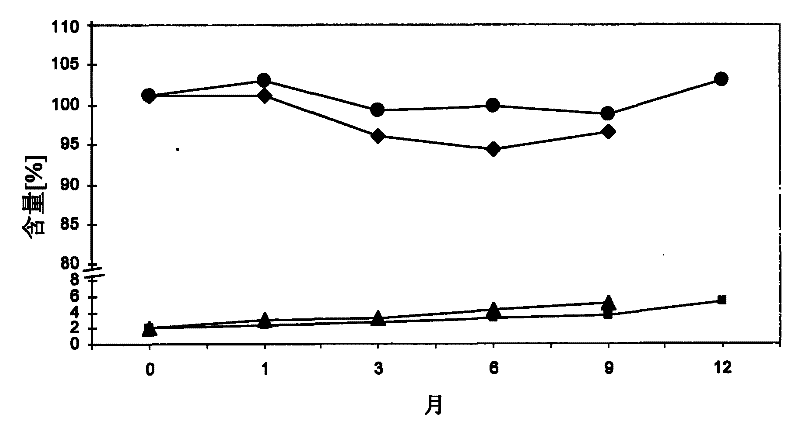
[0181] To test the stability of calcium 5-methyl-(6S)-tetrahydrofolate during storage under various conditions. The following stability data were obtained upon storage at 25°C / 60%RH and 40°C / 75%RH respectively (see Tables 1 and 2 below). Stability was tested in open and closed containers.
[0182] Table 1: Percentage of total degradation products
[0183]
[0184] Table 2: Amount (%)
[0185]
[0186] It can be seen that satisfactory stability of calcium 5-methyl-(6S)-tetrahydrofolate was obtained at 25°C even under conditions that allowed the tablets to be exposed to open air. In addition, satisfactory stability was obtained at 40°C (closed container), while 5-methyl-(6S)-tetrahydrofolate calcium was seen when the tablets were stored at 40°C while being exposed to open air. Significantly degra...
Embodiment 2
[0188] Embodiment 2-direct compression tablet; (Lactose Monohydrate / Cellulose Powder)
[0189] 80 mg tablet cores with the following composition are prepared by direct compression:
[0190]
[0191] To test the stability of calcium 5-methyl-(6S)-tetrahydrofolate during storage under various conditions. The following stability data were obtained upon storage at 25°C / 60%RH and 40°C / 75%RH respectively (see Tables 3 and 4 below). Stability was tested in open and closed containers.
[0192] Table 3: Sum of decomposition products (%)
[0193]
[0194] Table 4: percentage amount
[0195]
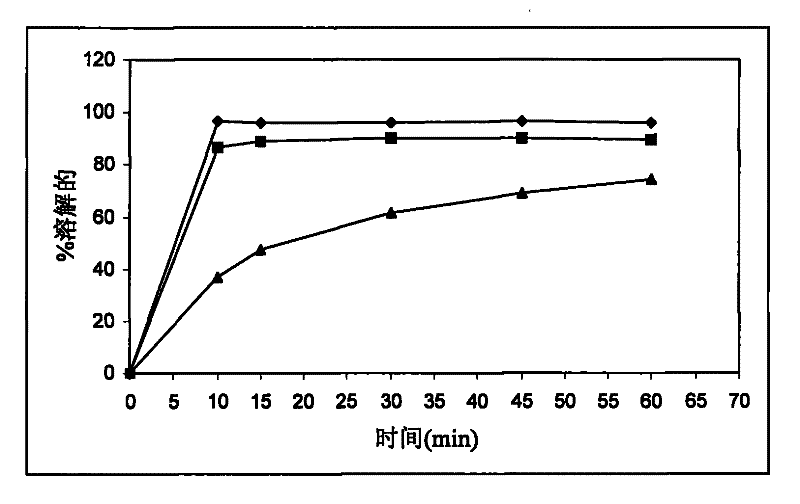
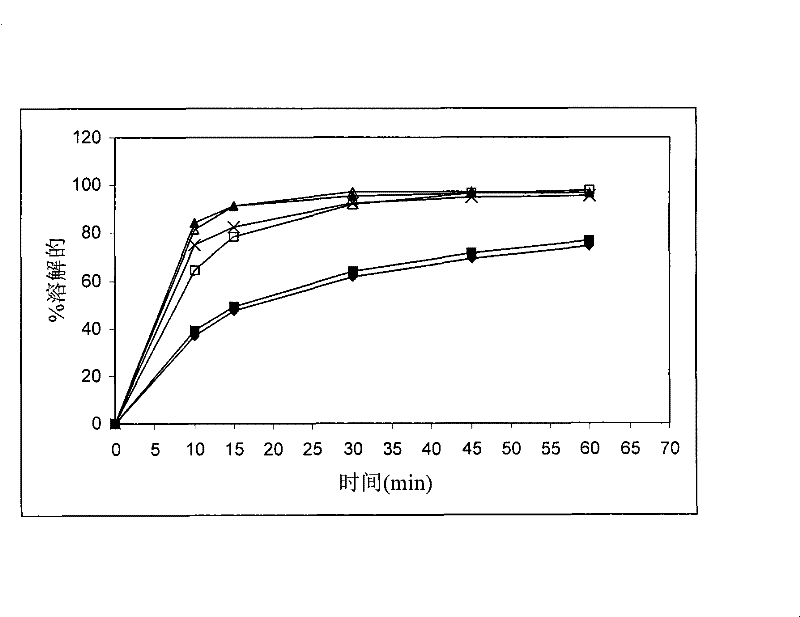
[0196] It can be seen that satisfactory stability of calcium 5-methyl-(6S)-tetrahydrofolate was obtained at 25°C. However, like Example 1, the dissolution rate of drospirenone was unsatisfactorily slow.
Embodiment 3
[0197] Embodiment 3 - direct compression tablet; (lactose monohydrate)
[0198] 80 mg tablet cores with the following composition are prepared by direct compression:
[0199]
[0200] The stability of calcium 5-methyl-(6S)-tetrahydrofolate was found to be unsatisfactory when stored at 25°C / 60%RH and 40°C / 75%RH, respectively. Like Examples 1 and 2, the dissolution rate of drospirenone was unsatisfactorily slow.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com