Antibodies and immunoconjugates and uses therefor
A technology for antibodies and biological samples, applied in the direction of anti-animal/human immunoglobulin, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, non-active ingredient medical preparations, etc., can solve the problem of efficacy low level problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
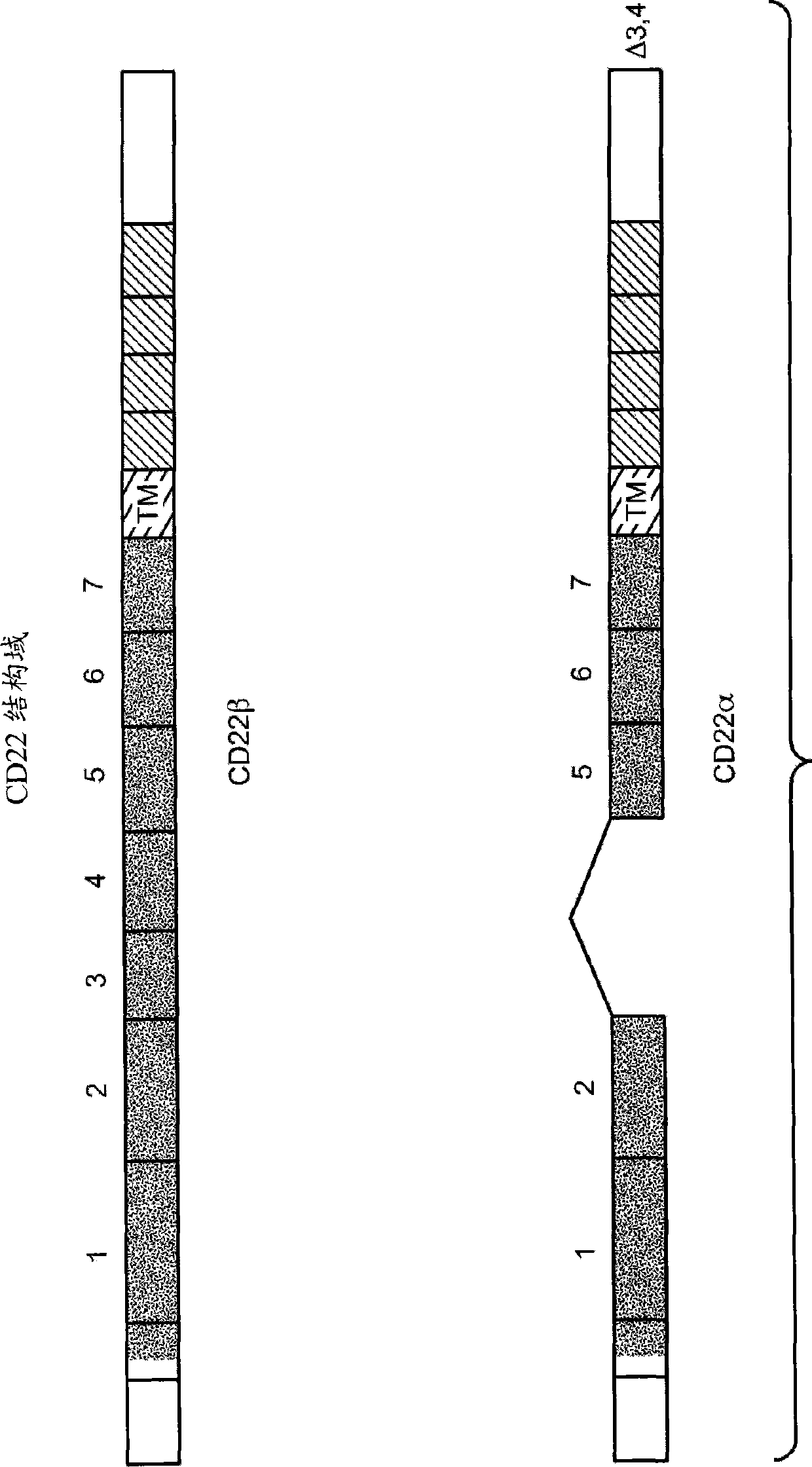
Image
Examples
Embodiment 1
[0984] Example 1: Preparation of mouse anti-human CD22 monoclonal antibody
[0985]A murine monoclonal antibody capable of specifically binding to human CD22 was prepared. Six-week-old BALB / c female mice were immunized in their paw pads with purified human CD22 plus his-8-tagged extracellular domain (SEQ ID NO: 30( ECD) at the C-terminus plus the sequence GRAHHHHHHHH) or the extracellular domain of CD22 plus his-8 tag comprising domains 1-7 (SEQ ID NO: 28 (ECD) plus the His sequence tag above). Subsequent injections were administered in the same manner one and three weeks after the initial immunization. Three days after the final injection, inguinal and popliteal lymph nodes were removed and pooled, and single cell suspensions were prepared by passing the tissue through a steel gauze. The cells were fused with mouse myeloma, such as P3X63-Ag8.653 (ATCCCRL1580), at a 4:1 ratio in high glucose (DMEM) containing 50% w / v polyethylene glycol 4000. Then with 2x10 5 Density per w...
Embodiment 2
[0988] Example 2: FACS-based assay for the analysis of anti-human CD22 monoclonal antibodies (MAbs)
[0989] CHO cells expressing human CD22 on their surface were incubated with anti-CD22 hybridoma supernatant in 100 μl FACS buffer (0.1% BSA in PBS, pH 7.4, 10 mM sodium azide) at 4° C. for 30 minutes, followed by Wash once with FACS buffer. The amount of anti-CD22 binding was determined by aliquots of the antibody / cell mixture with polyclonal FITC-conjugated goat or rabbit anti-mouse IgG (AccurateChem. Co., Westbury, NY) (for murine test antibody ) or goat or rabbit anti-human IgG (for humanized antibodies) were incubated at 4°C for 30 minutes, followed by three washes with FACS buffer.
Embodiment 3
[0990] Example 3: Preparation of humanized anti-CD22 antibody
[0991] A humanized 10F4 antibody was generated in which the hypervariable region (HVR) amino acid residues (interchangeably referred to as complementarity-determining regions) were modified by site-directed mutagenesis (Kunkel et al., Methods Enzymol. (1987), 154:367-382). or CDR) to obtain two variants, humanized 10F4v1 and humanized 10F4v2 (also referred to herein as "10F4v1" or "hu10F4v1" or "10F4v2" or "hu10F4v2", respectively). A third version, humanized 10F4v3 ("10F4v3" or "hu10Fv3") used in some of the studies disclosed herein, has the same mature protein light and heavy chain amino acid sequences as The expression vectors contain different signal sequences.
[0992] Humanization of the murine 10F4 antibody was performed as disclosed herein. Briefly, the light and heavy chain hypervariable regions of murine 10F4 were cloned into a modified consensus framework sequence to generate Figure 2A with 2B Amin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com