Recombinant CREG protein and application thereof
A recombinant protein and protein technology, applied in the field of recombinant CREG protein and its application, can solve the problem of low CREG protein production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] The following examples are provided to further explain and illustrate the present invention. Example:
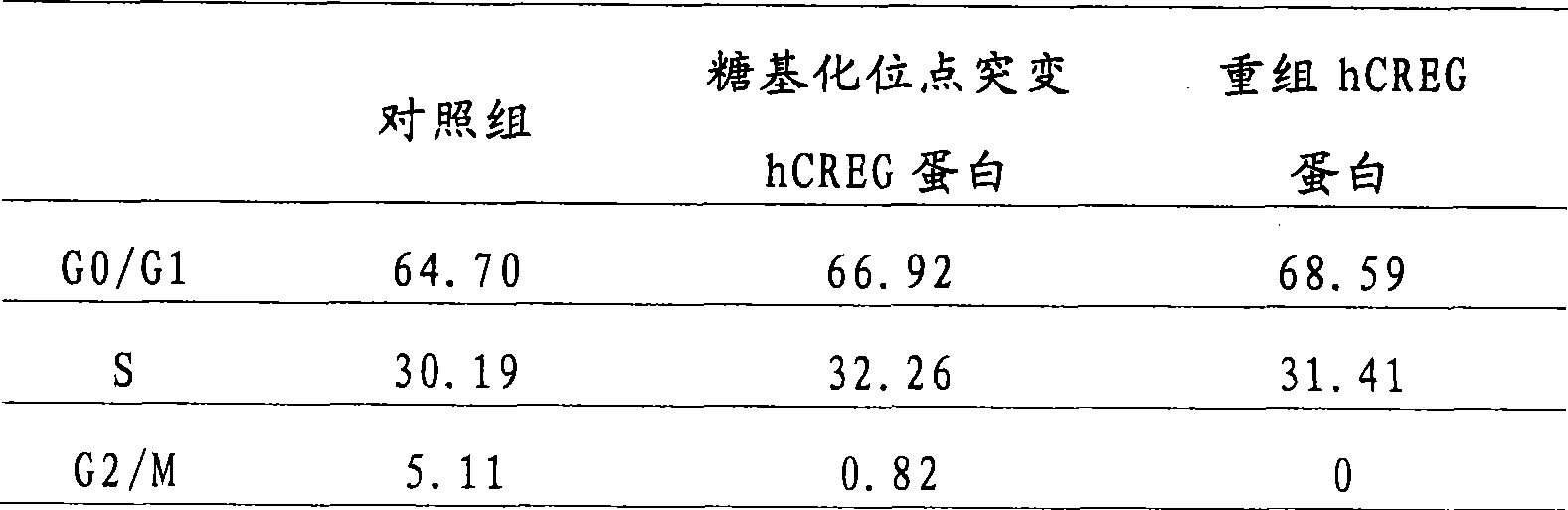
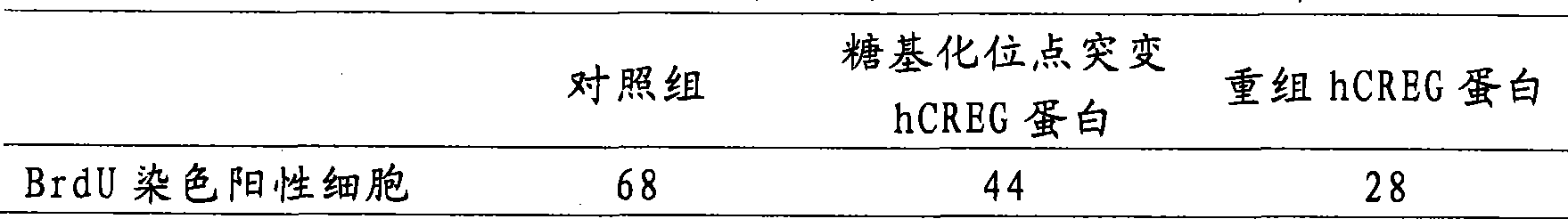
[0023] 1. Construction and identification of eukaryotic expression vector pcDNA3.1myc-His / hCREG (including hCREG and hCREG with glycosylation site mutation)
[0024] According to the open reading frame in the hCREG cDNA sequence registered in GenBank (NM003851), hCREG RT-PCR primers with stop codon mutations were designed and synthesized.
[0025] The upstream primer is: 5'-aa ggatcc atggccgggctatcccgc-3'
[0026] Basic Tm=66°C, salt (50mM) adjusted Tm=74°C;
[0027] The downstream primer is: 5'-gc gaattc Gcactgaactgtgacatttaatattcttctgg-3'
[0028] Basic Tm=64°C, salt (50mM) adjusted Tm=74°C;
[0029] The uppercase letters represent the stop codons of C / G mutations.
[0030] Extract mRNA from VSMCs cultured in vitro and reverse transcribe into cDNA (cDNA first-strand synthesis kit from TakaRa Company. Amplification conditions are: 25°C, 5 minutes; 42°C, 50 minut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com