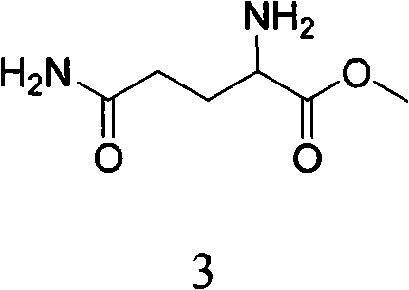
Method for preparing lenalidomide
A technology of reflux temperature and reaction temperature, applied in the direction of organic chemistry, etc., can solve the problems of long steps, high risk, and difficulty in industrialization, and achieve the effects of low production cost, no three wastes, and short reaction steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
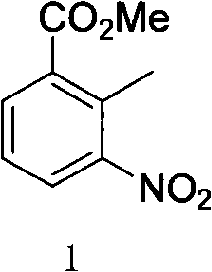
[0035] (1) Preparation of 2-methyl-3-nitrobenzoic acid methyl ester
[0036] 18.1g (0.1mol) of 2-methyl-3-nitrobenzoic acid was dissolved in 200ml of anhydrous methanol, and 11.9g (0.1mol) of thionyl chloride was added dropwise at a temperature below 0°C. After the dropping was completed, heat and reflux for 1 hour . Evaporate methanol to dryness, pour into ice water, adjust the pH value to 7-9, stir for 2-3 small test, a yellow solid is precipitated, filtered and dried to obtain 18g of product with a yield of 92.3%.
[0037] 1 H NMR (300MHz, CDCl3) δ: 2.6(s, 3H), 3.95(s, 3H), 7.35(m, 1H), 7.90(m, 2H)
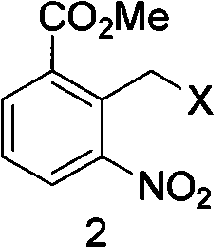
[0038] (2) Preparation of 2-chloromethyl-3-nitrobenzoic acid methyl ester
[0039] Dissolve 3.9 g (0.02 mol) of methyl 2-methyl-3-nitrobenzoate in 50 ml of chloroform, add 5.3 g (0.04 mol) of NCS (chlorosuccinimide), and heat to reflux for 24 hours. After cooling, add 100ml of water, separate the layers, extract the aqueous phase twice with 20ml of chloroform, combine the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com