Insoluble pharmaceutical composition
A technology for insoluble drugs and compositions, applied in drug combinations, antineoplastic drugs, pharmaceutical formulations, etc., can solve the problems of cumbersome process, difficult dissolution, high equipment requirements, etc., and achieve simple preparation process, improved dissolution rate, and convenient operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
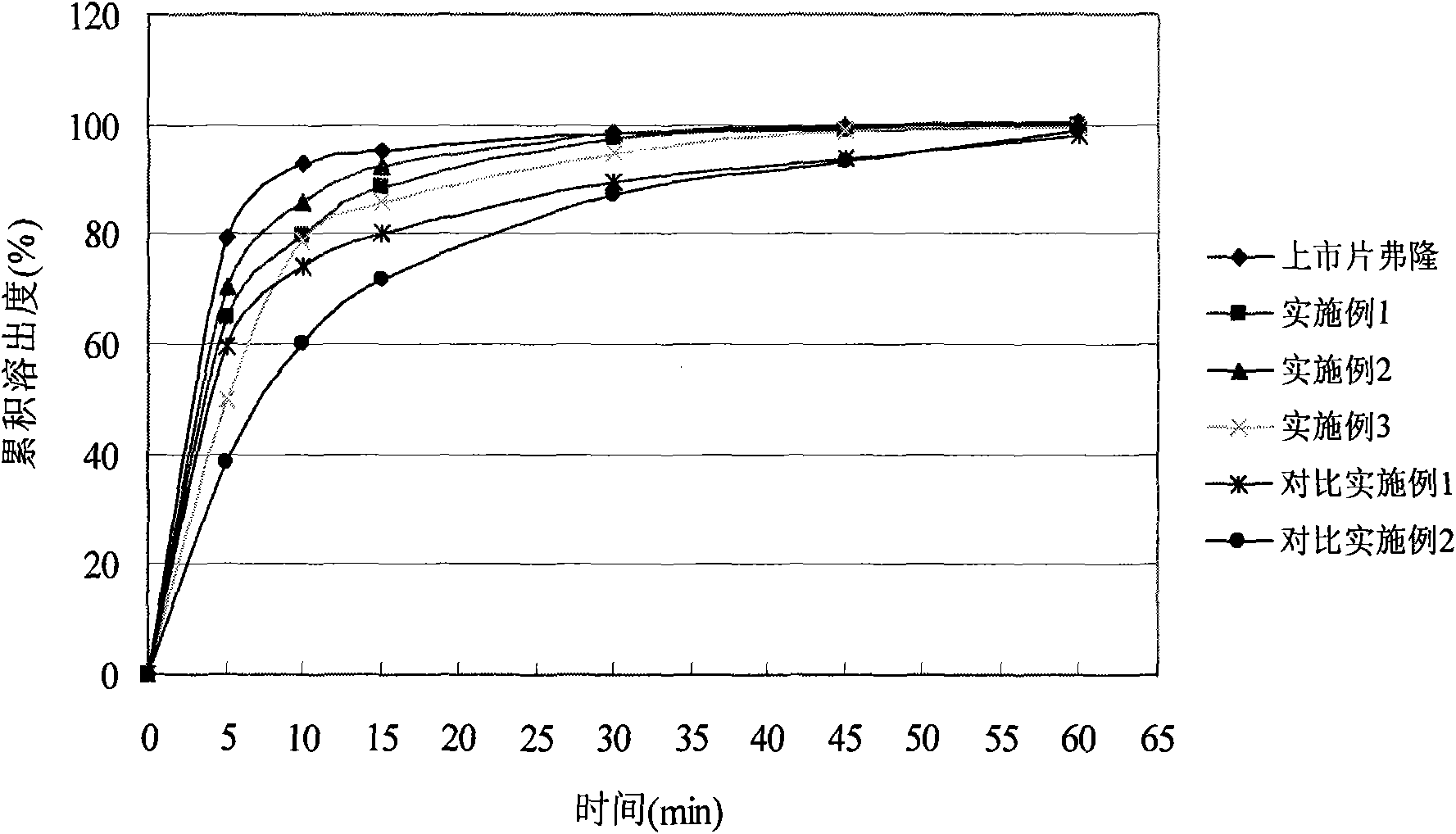
Image
Examples
Embodiment 1
[0012] Embodiment 1: Letrozole Granules
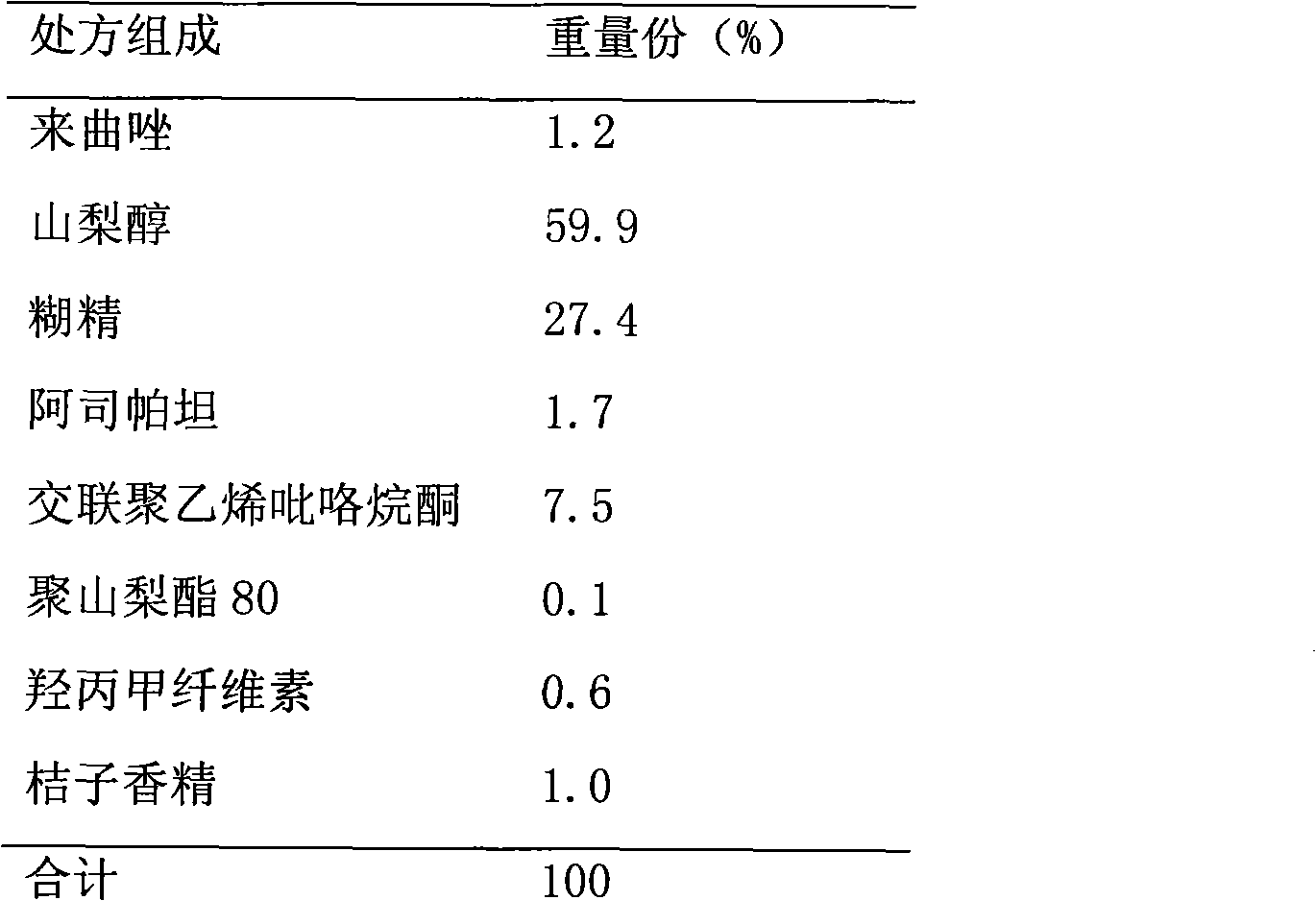
[0013] prescription:
[0014]
[0015] Preparation:
[0016] (1) Letrozole is pulverized through a 170 mesh sieve for subsequent use;
[0017] (2) All auxiliary materials are passed through an 80-mesh sieve for subsequent use;
[0018] (3) Sorbitol, dextrin, aspartame, and cross-linked polyvinylpyrrolidone are weighed and mixed uniformly to obtain material I. Letrozole is taken in an equal amount and added to material I in equal amounts, mixed uniformly to obtain material II;
[0019] (4) Add polysorbate 80 to 2% hypromellose aqueous solution to make a binder, add an appropriate amount of binder to material II to make a suitable soft material, granulate through a 24-mesh sieve, and ventilate at 50°C Dry, control the moisture to be less than 3%, and granulate with a 20-mesh sieve.
[0020] (5) Add orange essence to the dry granules, mix evenly, and pack.
Embodiment 2
[0021] Embodiment 2: letrozole tablet
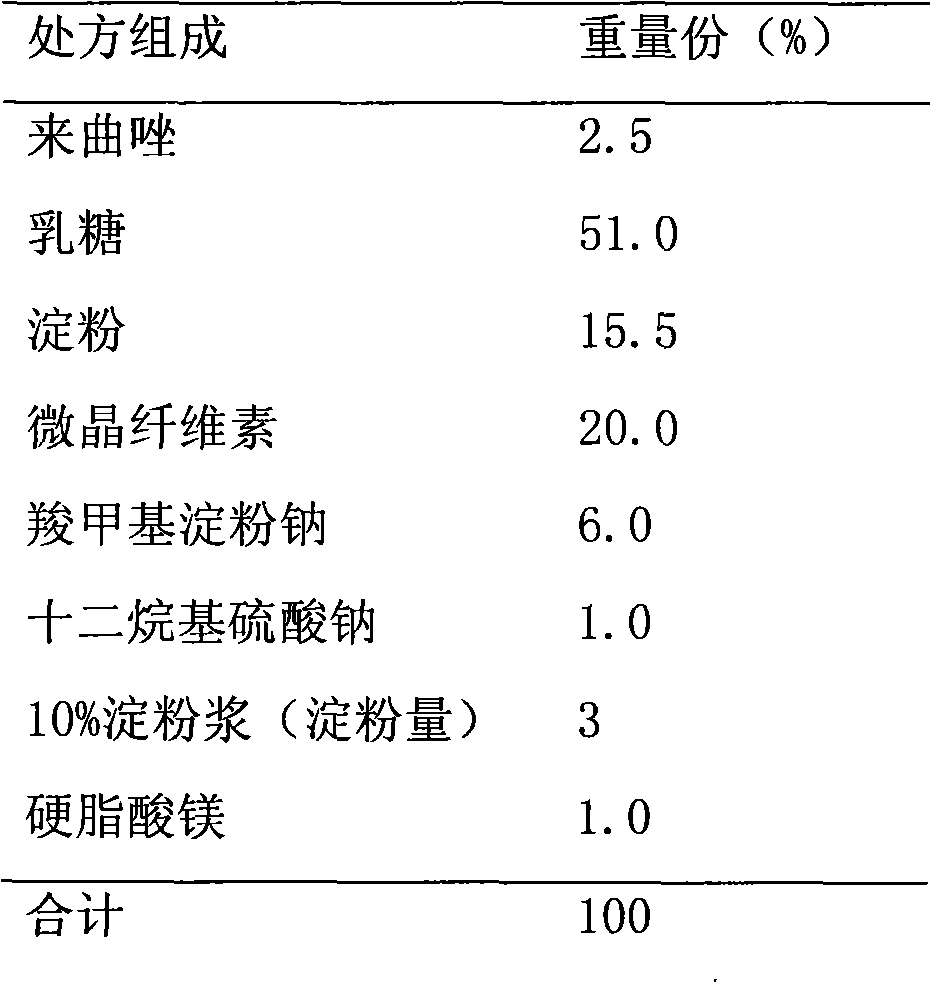
[0022] prescription:
[0023]
[0024] Preparation:
[0025] (1) Letrozole is pulverized through a 200-mesh sieve for subsequent use;
[0026] (2) All auxiliary materials are passed through a 100-mesh sieve for subsequent use;
[0027] (3) Lactose, starch, microcrystalline cellulose, sodium carboxymethyl starch that take by weighing recipe quantity mix homogeneously, obtain material I, take by weighing letrozole and sodium lauryl sulfate of recipe quantity mix, add material in equal amount I, mix homogeneously, obtain material II;
[0028] (4) Add appropriate amount of material II to 10% starch slurry to make a suitable soft material, pass through a 20-mesh sieve to granulate, ventilate and dry at 50°C, control the moisture content to be less than 3%, and granulate with a 20-mesh sieve.
[0029] (5) Add magnesium stearate to the dry granules, mix evenly, compress into tablets, and pack.
Embodiment 3
[0030] Embodiment 3: Letrozole capsules
[0031] prescription:
[0032]
[0033] Preparation:
[0034] (1) Letrozole is pulverized through a 150-mesh sieve for subsequent use;
[0035] (2) All auxiliary materials are passed through an 80-mesh sieve for subsequent use;
[0036] (3) Mannitol, pregelatinized starch, croscarmellose sodium, and low-substituted hydroxypropyl cellulose fillers are weighed and mixed uniformly to obtain material I, and letrozole and poloxa are taken in prescription quantities Mud and Polyethylene Glycol 6000 were mixed evenly, and the same amount was added to the material I, and the mixture was evenly mixed to obtain the material II;
[0037] (4) Fill capsules with the material II, and pack.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com