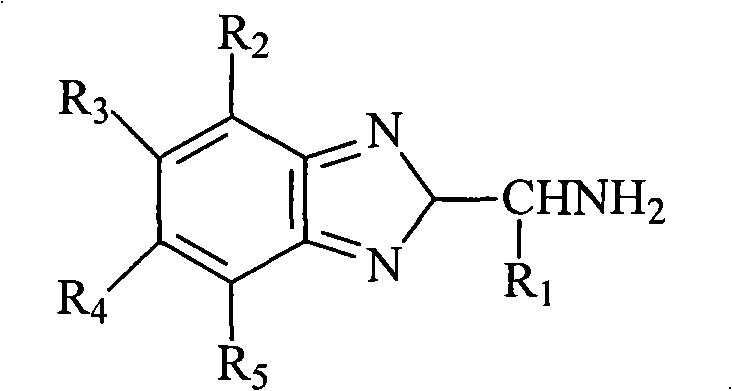
Method for preparing 2-aminoalkylbenzimidazole derivatives
A technology for aminoalkylbenzimidazole and derivatives, which is applied in the field of preparation of 2-substituted benzimidazole derivatives, and can solve problems such as not obtaining target compounds and having no literature reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0015] Example 1:
[0016] Add 1.0g glycine, 1.04g o-phenylenediamine, 10ml 85% phosphoric acid, 30ml polyphosphoric acid to a three-necked flask equipped with a thermometer, reflux condenser and stirrer, and heat the reaction in a silicon oil bath to control the reaction temperature at 185°C. The reaction time was 16 hours, and the color of the reaction solution changed from colorless to dark gray and finally to dark blue. After the reaction, the reaction mixture was cooled to room temperature and transferred to a 500 ml beaker. After dilution with 200ml of water, 10% NaOH was slowly added dropwise to neutralize to pH=8, a light gray flocculent precipitate was precipitated, vacuum filtration, the obtained precipitate was washed three times with cold water to obtain a crude product. It was recrystallized with absolute ethanol and decolorized by activated carbon to obtain 1.29 g of light pink powder crystals with a yield of 87.0%. M.P: 228-233℃; IR: 3300cm -1 (N-H), 3055cm -1 (A...
Example Embodiment
[0017] Example 2:
[0018] Add 4.14g L-tyrosine, 2.4g o-phenylenediamine, 20ml 85% phosphoric acid, 50ml polyphosphoric acid into a three-necked flask equipped with thermometer, reflux condenser and stirrer, and heat the reaction in an oil bath to control the reaction temperature At 192°C, the reaction time was 20 hours. After the reaction, the reaction mixture was cooled to room temperature and transferred to a 500 ml beaker. After dilution with 200ml of water, 10% NaOH was slowly added dropwise to neutralize to pH=8, and a dark brown flocculent precipitate was precipitated, which was vacuum filtered, and the obtained precipitate was washed with cold water three times to obtain a crude product. It was recrystallized with absolute ethanol and decolorized by activated carbon to obtain 3.96 g of light brown powdery crystals with a yield of 79.2%. M.P: 233-237°C; IR: 3436cm -1 (N-H), 3100-3650cm -1 (O-H), 1690cm -1 (C=N), 1600-1500cm -1 Four bands (aromatic ring overtone), 1380cm -...
Example Embodiment
[0019] Example 3:
[0020] Add 8.64g of chloroacetic acid and 6.62g of o-phenylenediamine, 20ml of 85% phosphoric acid, 40ml of polyphosphoric acid to a three-necked flask equipped with a thermometer, reflux condenser and stirrer. Heat the reaction in a silicon oil bath to control the reaction temperature of 140- At 145°C, the reaction time was 12 hours, and the color of the reaction solution gradually changed from colorless to light yellow. After the reaction, the reaction mixture was cooled to room temperature and transferred to a 500 ml beaker. After dilution with 200 ml of water, 10% NaOH was slowly added dropwise to neutralize to pH=8, a yellow precipitate was precipitated, which was vacuum filtered, and the obtained precipitate was washed three times with cold water to obtain a crude product. It was recrystallized with absolute ethanol and decolorized by activated carbon to obtain light yellow needle-like crystals with a yield of 82.6%. M.P: 146-150℃; IR: 3458cm -1 (N-H),...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap