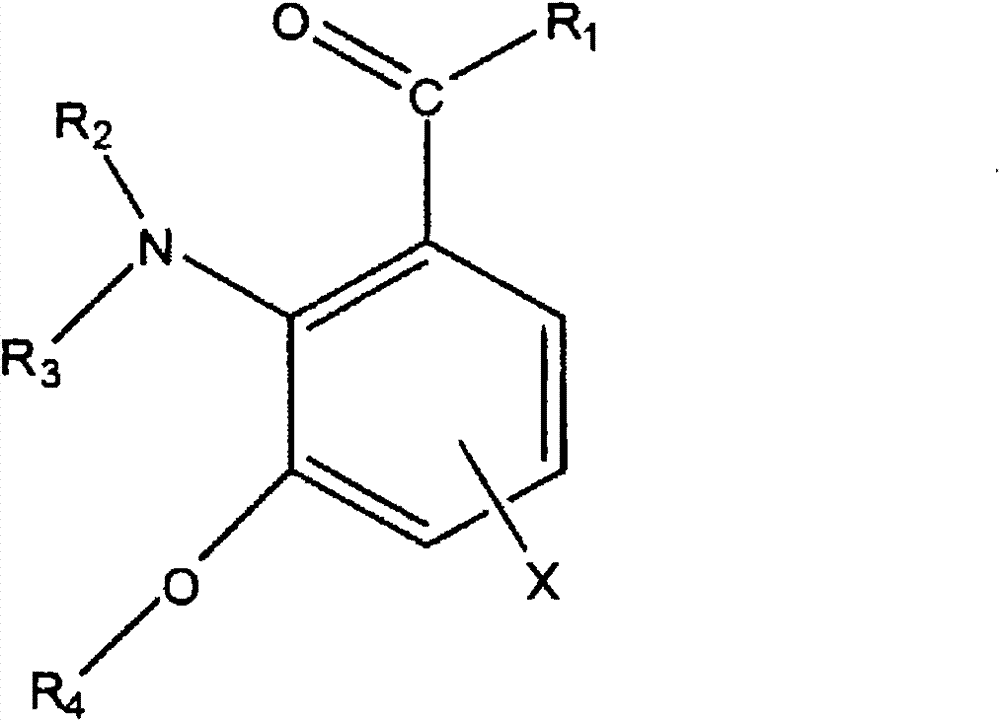
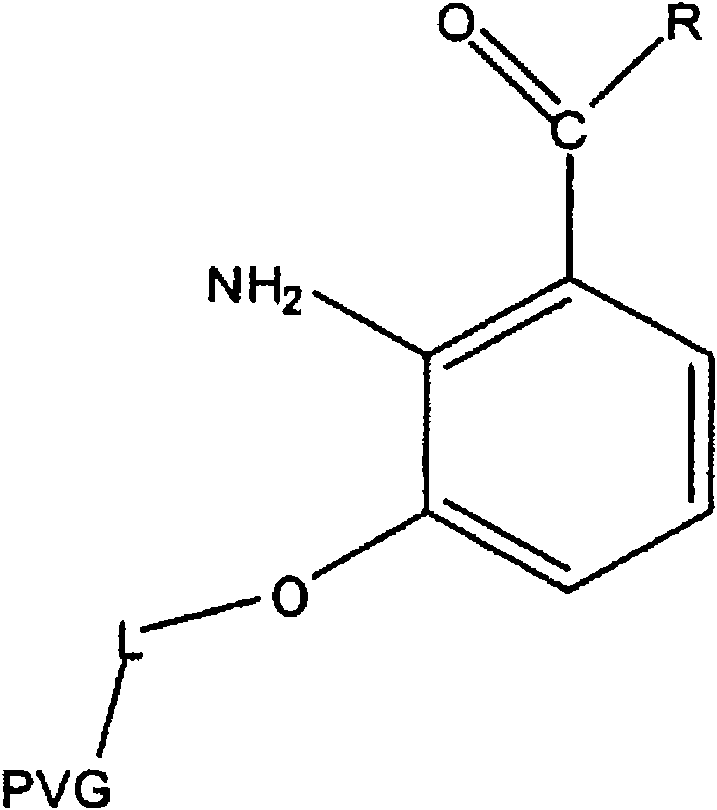
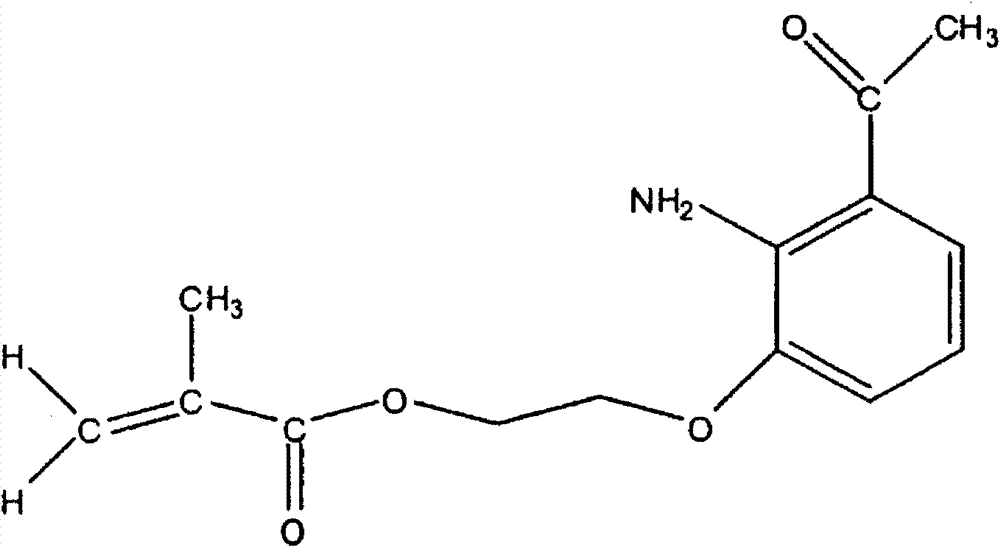
Light filters comprising a naturally occurring chromophore and derivatives thereof
A technology of chromophores and derivatives, applied in optics, optical components, instruments, etc., can solve the problems of not providing versatility and adjustability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Embodiment 1 prepares 2-chloroethyl methacrylate
[0121]
[0122] A solution of 530 ml (7.91 mol) of 2-chloroethanol and 1210 ml (14.96 mol) of pyridine in 3LEtOAc was cooled to 5° C. in an ice bath and 820 ml (6.71 mol) of acryloyl chloride (tech., 80 %) in 1 L of EtOAc such that the internal temperature was kept below or equal to 17 °C. This took 2.5 hours. The reaction mixture was slowly cooled to room temperature overnight. The slurry was then filtered and the filter cake of pyridine hydrochloride was washed with about 3 L of EtOAc. The filtrate was divided into 3 approximately equal fractions and each fraction was thus refined. Each was extracted with 2 x 1 L of 2N HCI, 2 x 500 ml of DI water and 1 x 500 ml of 5 wt% sodium bicarbonate, then the three fractions were combined and dried over anhydrous sodium sulfate (764 g). After filtration, the EtOAc solution was treated with 11.1 g hydroquinone and concentrated in vacuo to yield 1245.7 g pale yellow oil. ...
Embodiment 2
[0123] Example 2 Preparation of kynurenine chromophore compounds
[0124]
[0125] A reaction mixture containing 103.0 g (681 mmol) of 2-amino-3-hydroxyacetophenone, 125.1 g (842 mmol) of 2-chloroethyl methacrylate and 122.6 g (887 mmol) of potassium carbonate in 930 ml of DMSO was processed for 5 hours Warm to 85°C and stir overnight (16 hours) at this temperature. After this time, TLC (silica gel, hexane:acetone 3:1) showed the reaction was complete. After cooling to 48 °C, the reaction mixture was partitioned between 2 L of DI water and 500 ml of toluene. After a second 500 ml toluene extraction of the aqueous phase, the combined organic layers were washed twice with 1 L of 10 wt% potassium carbonate. The organic phase was then passed through a 1253 g silica gel (70-230 mesh) plug, eluting with 3:1 hexane:acetone until no more product was visible (TLC) leaving the plug. This used 6 L of elution solvent. The eluate was concentrated under vacuum to yield 199 g of amb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com