Quinoline carboxylic acid derivative containing isatin substitute and preparation method thereof
A technology of oxoquinoline and carboxylic acid, applied in the field of medicinal chemistry
Inactive Publication Date: 2010-08-25
MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI +1
View PDF4 Cites 5 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, so far there is no literature report on further research on the anti-tuberculosis effect of baloxacin
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
| Mp | aaaaa | aaaaa |
Login to View More
Abstract
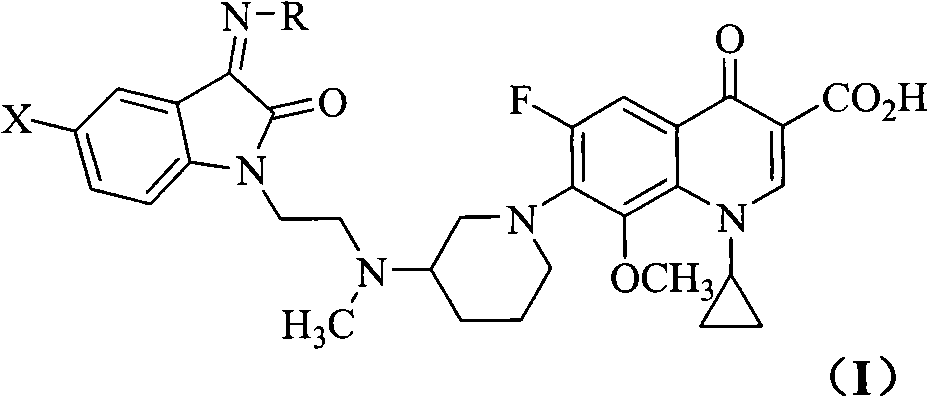
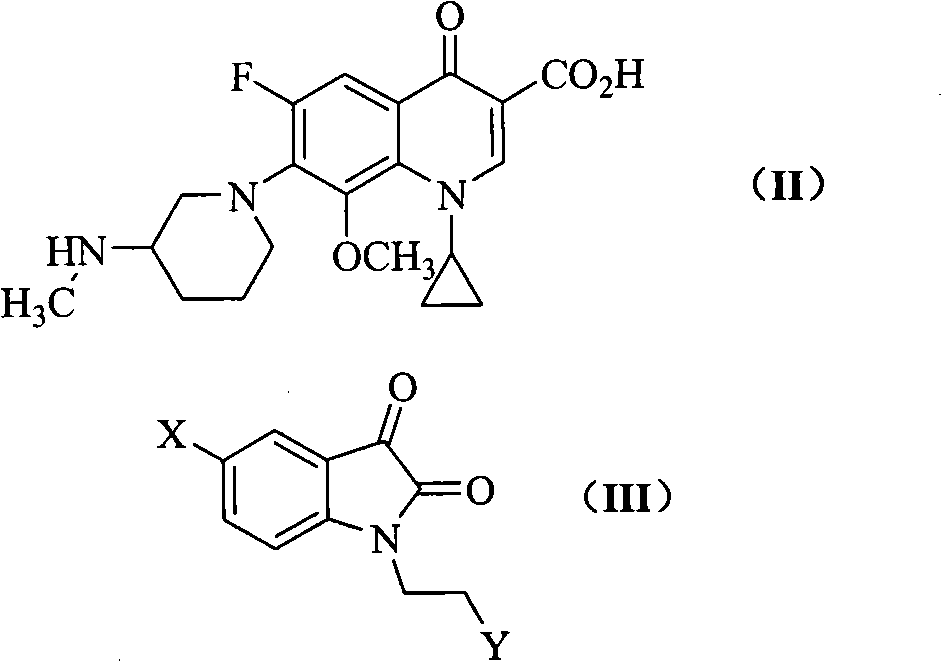
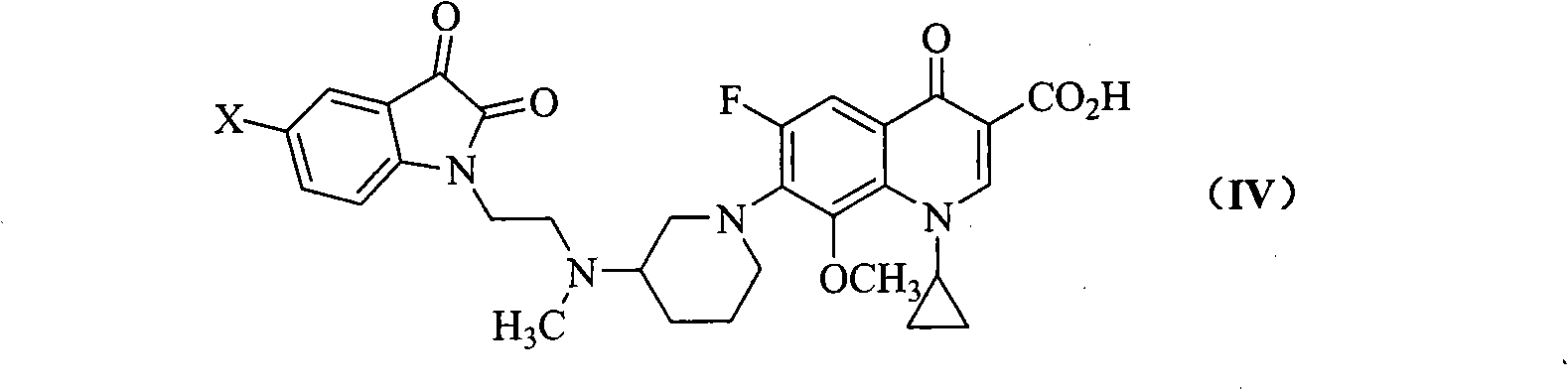
The invention relates to a quinoline carboxylic acid derivative containing isatin substitute, a preparation method, medical purposes, and an anti-tuberculosis drug combination containing the quinoline carboxylic acid derivative. More specifically, the invention relates to a new fluoroquinolone carboxylic acid derivative. The 7-site of the derivative on a quinoline core is 3-[N- methyl-N-2-(beta-substitute imino isatin-1-radical) ethyl] amino-1- piperidyl, the 8-site is methoxy group, and the 1-site is cyclopropyl. Compared with the traditional clinical anti-tuberculosis drug of a fluoroquinolone class (such as Ciprofloxacin), the quinoline carboxylic acid derivative has more excellent anti-tuberculosis mycobacterium activity.
Description
technical field The invention belongs to the field of medicinal chemistry, and relates to quinoline carboxylic acid derivatives with excellent anti-tuberculosis activity and a preparation method thereof, and an anti-tuberculosis pharmaceutical composition containing them; star derivatives. Background technique Tuberculosis (TB) is one of the major infectious diseases caused by Mycobacterium tuberculosis (MTB), which seriously endangers human health. Since the 1980s, the incidence of drug-resistant TB, especially multi-drug-resistant TB (MDR-TB), has been increasing and the combination of TB and HIV / AIDS has caused the TB epidemic to rise again, becoming a major public health problem and a global concern. Social Issues. According to statistics, there are 8 million new TB patients every year in the world, nearly 3 million people die of tuberculosis, and nearly 1 / 3 of the population carries latent Mycobacterium tuberculosis, which is a potential risk of disease. Unfortunatel...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D401/14A61K31/4709A61P31/06
Inventor 郭慧元刘明亮冯连顺刘秉全张婷婷徐静静
Owner MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com