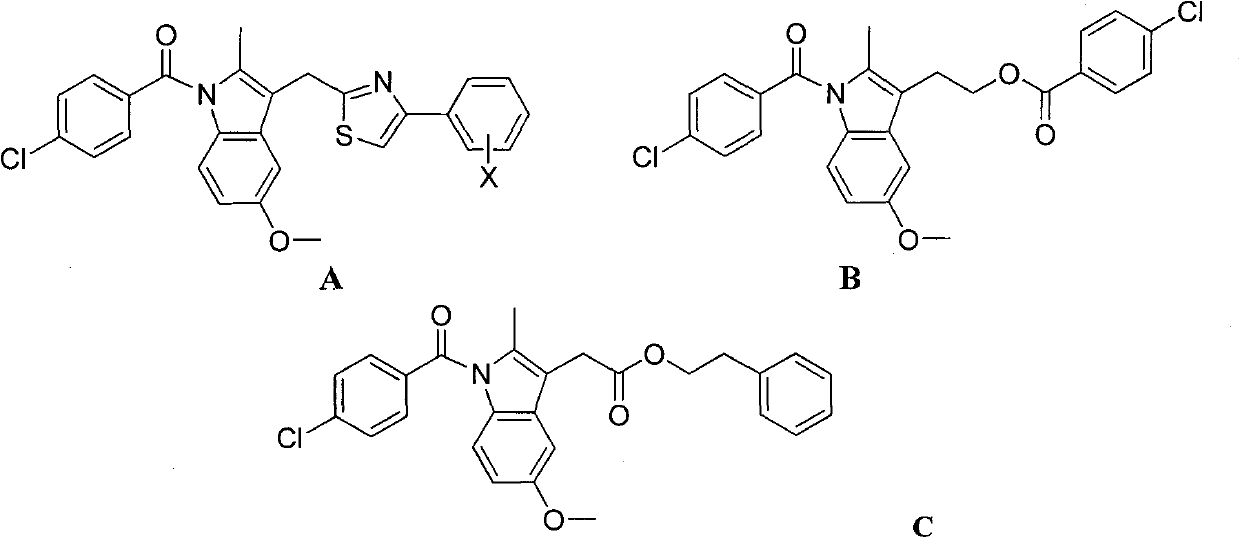
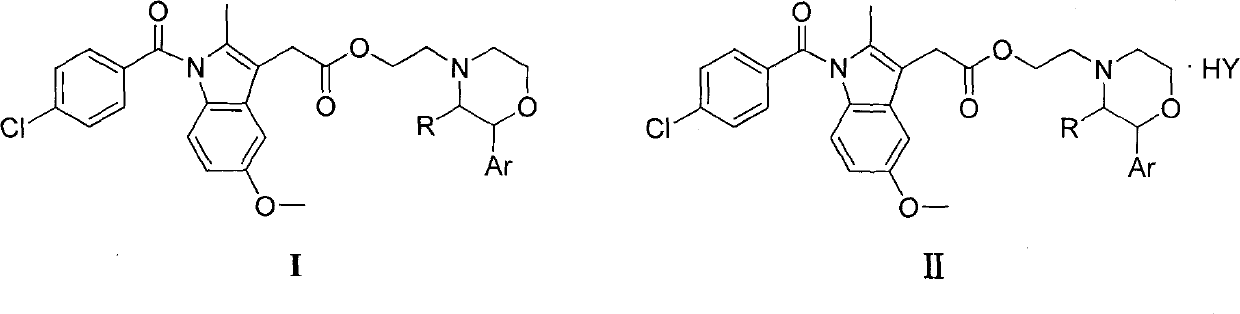
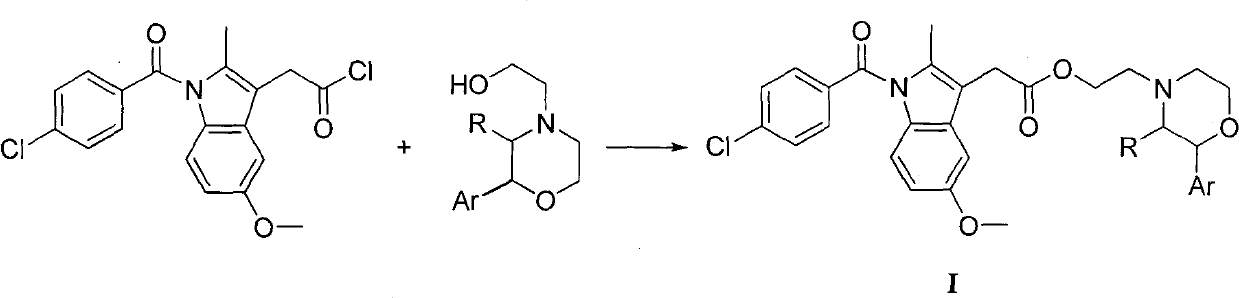
Indometacin 2-arylmorpholine ethyl, preparation method and application thereof
A technology of aryl morpholino ethyl ester and aryl morpholino ethyl ester, which is applied in the field of new compounds and their preparation, and can solve problems such as gastrointestinal side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 The preparation of indomethacin 2-(2-phenylmorpholinyl) ethyl ester and its hydrochloride
[0026] (1) Preparation of Indomethacryloyl Chloride
[0027] Dissolve 3 mmol of indomethacin in 8 mL of dichloromethane, add 1 mL of oxalyl chloride, stir in an ice bath for 3 h, evaporate the solvent under reduced pressure, and separate the residual oxalyl chloride to obtain indomethacin as a yellow solid.
[0028] (2) Preparation of indomethacin 2-(2-phenylmorpholinyl) ethyl ester and its hydrochloride
[0029]
[0030] Dissolve indomethanoyl chloride prepared in 8 mL of tetrahydrofuran, add dropwise 2 mmol of 2-phenyl-4-hydroxyethylmorpholine in tetrahydrofuran, 1 mL of triethylamine as an acid-binding agent, stir at room temperature for 6 hours, and filter after completion of the reaction. The filtrate was decompressed to remove the solvent tetrahydrofuran, washed away excess indomethacin with 1.0mol / L sodium hydroxide solution, extracted with ethyl acetate t...
Embodiment 2
[0032] Embodiment 2 Preparation of indomethacin 2-[2-(4-methylphenyl)morpholinyl] ethyl ester and hydrochloride thereof
[0033]
[0034]
[0035] Dissolve the obtained indomethacryloyl chloride in 8 mL of tetrahydrofuran, add dropwise 2 mmol of 2-(4-methylphenyl)-4-hydroxyethylmorpholine in tetrahydrofuran, 1 mL of triethylamine as an acid-binding agent, and stir at room temperature for 6 h , the reaction was completed, filtered, and the filtrate was decompressed to remove the solvent tetrahydrofuran, washed off excess indomethacin with 1.0mol / L sodium hydroxide solution, extracted with ethyl acetate to obtain an organic layer, evaporated to remove ethyl acetate, and column chromatography Indomethacin 2-[2-(4-methylphenyl)morpholinyl]ethyl ester.
[0036] Dissolve indomethacin 2-[2-(4-methylphenyl)morpholinyl]ethyl ester with a small amount of anhydrous ether, and pass through dry hydrogen chloride to obtain indomethacin 2-[2-(4-methylbenzene Base) morpholino] ethyl es...
Embodiment 3
[0037] Embodiment 3 Preparation of Indomethacin 2-[2-(4-ethylphenyl)morpholinyl]ethyl ester and its hydrochloride
[0038]
[0039] Dissolve the obtained indomethacryloyl chloride in 8 mL of tetrahydrofuran, add dropwise 2 mmol of 2-(4-ethylphenyl)-4-hydroxyethylmorpholine in tetrahydrofuran, 1 mL of triethylamine as an acid-binding agent, and stir at room temperature for 6 h , the reaction was completed, filtered, and the filtrate was decompressed to remove the solvent tetrahydrofuran, washed off excess indomethacin with 1.0mol / L sodium hydroxide solution, extracted with ethyl acetate to obtain an organic layer, evaporated to remove ethyl acetate, and column chromatography Indomethacin 2-[2-(4-ethylphenyl)morpholinyl]ethyl ester.
[0040] Dissolve indomethacin 2-[2-(4-ethylphenyl)morpholinyl]ethyl ester with a small amount of anhydrous ether, and pass through dry hydrogen chloride to obtain indomethacin 2-[2-(4-ethylphenyl Base) morpholino] ethyl ester hydrochloride, yiel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com