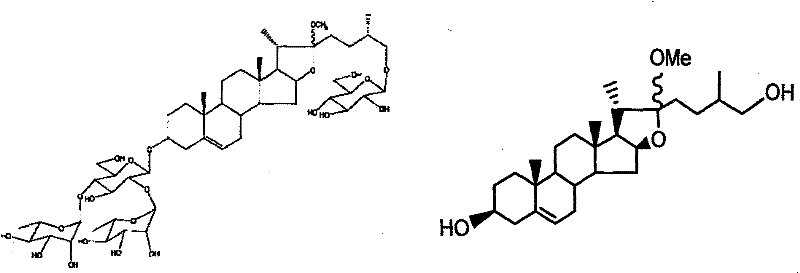
Convenient synthesis method of methylprotodioscin
A technique for the synthesis of methyl protodiosgenin, which is applied in the fields of steroids and organic chemistry, can solve the problems that the synthesis of methyl protodiosgenin has not been reported, and achieve the effect of cheap price and abundant resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
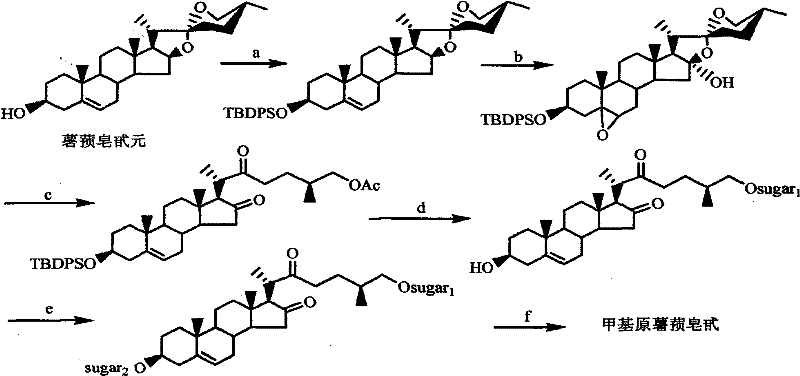
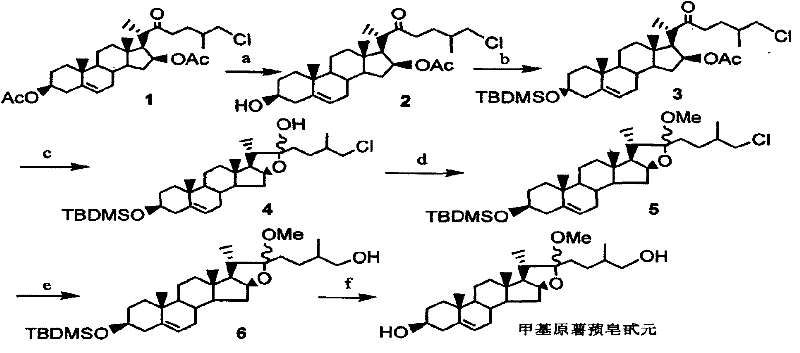
[0026] Step 1, Synthesis of 26-chloro-3β-hydroxyl-16β-acetoxy-5-cholesten-22-one 2
[0027] 11.0g (20mmol) compound 1 and 5.52g (40mmol) K 2 CO 3Place in 150 mL of a mixed solution of tetrahydrofuran and methanol (v:v=1:1), stir at room temperature, and monitor by TLC until the reaction is complete. The reaction takes about 3 hours. After the reaction is completed, add an appropriate amount of ethyl acetate and wash with saturated brine. The organic phase After drying with anhydrous sodium sulfate and removing the solvent under reduced pressure, 9.6 g of oil was obtained, which was purified by silica gel column chromatography to obtain 8.8 g of white solid compound. The yield was 87.0%.
[0028] Compound 2:C 29 h 45 ClO 4 (M=493.12), m.p: 167-168°C.
[0029] 13 C NMR (CDCl 3 , 75MHz) δ13.3 (C 21 ), 16.8 (C 18 ), 17.6 (C 27 ), 19.4 (C 19 ), 20.8 (CH 2 ), 21.2 (OAc), 27.6 (CH 2 ), 31.4(CH), 31.6(CH 2 ), 31.7 (CH 2 ), 34.9 (CH 2 ), 35.0 (CH), 36.5 (C10), 37.2 (CH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com