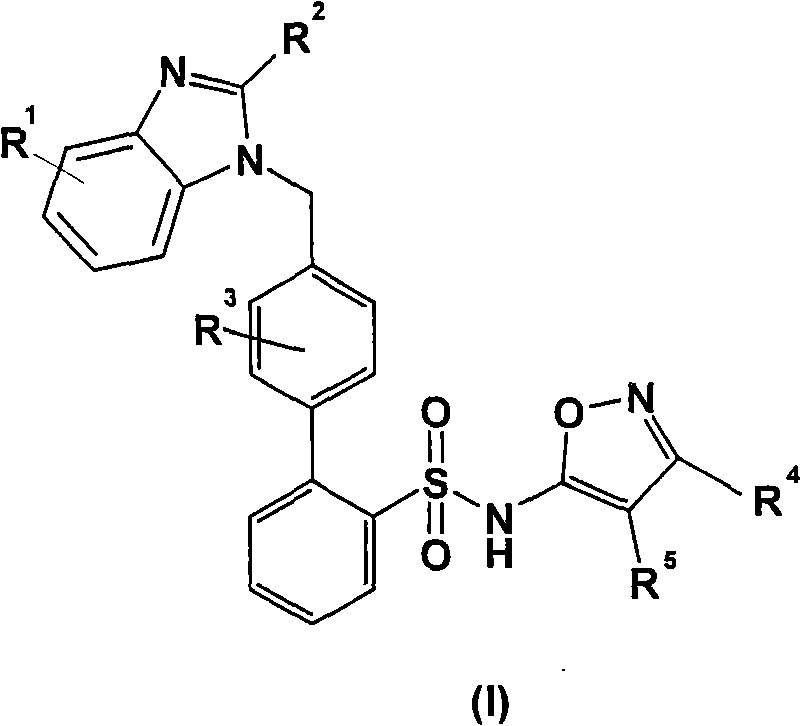
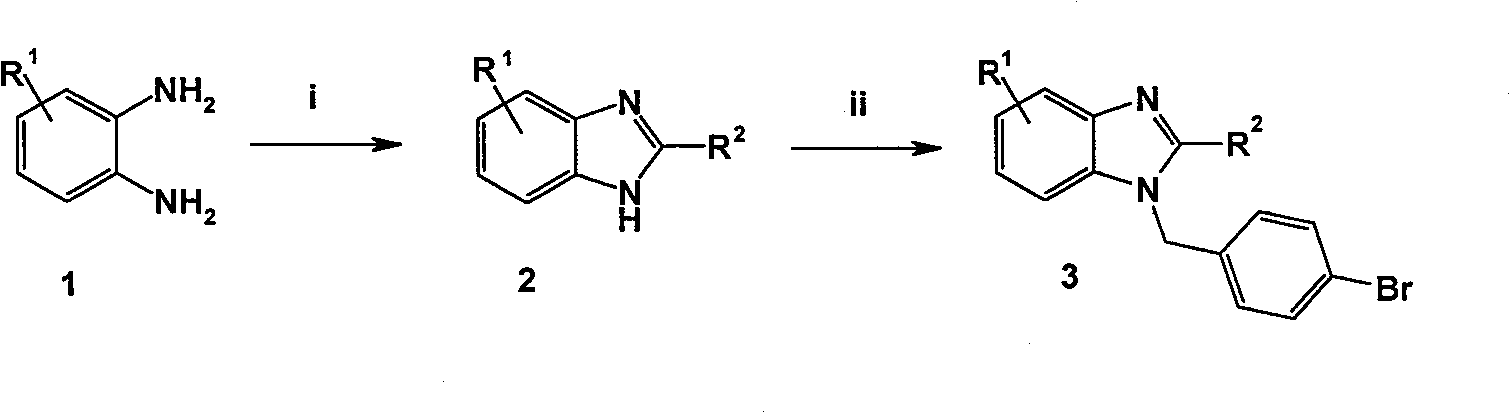
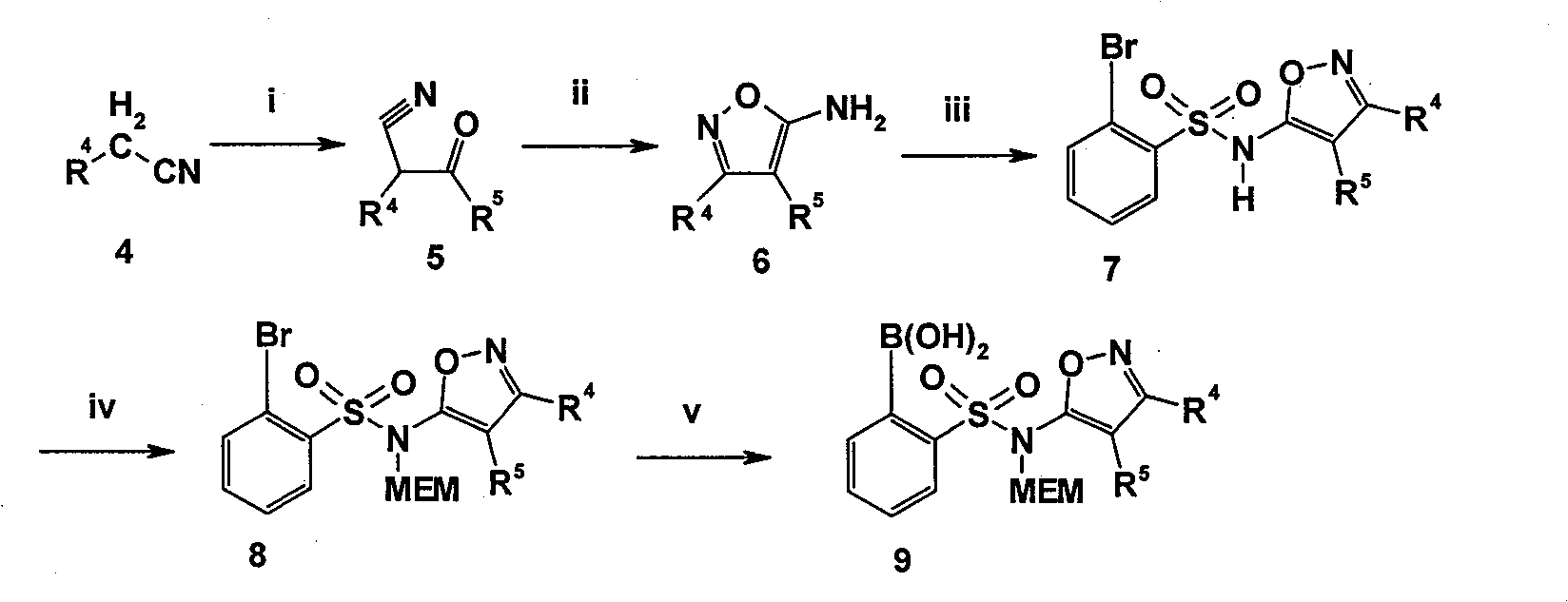
Benzimidazole derivative with cardiovascular activity and preparing method and application thereof
A compound and medicinal salt technology, applied in the fields of benzimidazole derivatives with cardiovascular activity, their preparation and application, can solve the problem that the condition has not been significantly improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1H-benzimidazole
[0057] Mix o-phenylenediamine (1) (5.40g, 0.05mol) and formic acid (2.64g, 0.06mol), add 4mol / L hydrochloric acid (60ml) to reflux for 2h, add ammonia water to adjust pH to alkaline, filter, wash with water, 95% Recrystallized from ethanol to obtain 2.65 g of white solid, yield: 45.0%, m.p.172-173°C. (Document: mp: 172-173℃)
Embodiment 2
[0059] 2-Methyl-1H-benzimidazole
[0060] Referring to the preparation method of 1H-benzimidazole, white crystals were obtained, yield: 53.7%, m.p.176-178°C. (Document: m.p.177-177.5℃)
Embodiment 3
[0062] 2-Ethyl-1H-benzimidazole
[0063] Referring to the preparation method of 1H-benzimidazole, white crystals were obtained, yield: 48.7%, m.p.173-175°C. (Document: m.p.174.5℃)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com