Antibodies against PD-1 and uses therefor
A PD-1 and antibody technology, applied in the direction of antibodies, applications, anti-inflammatory agents, etc., can solve problems such as weakening of T cell response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] Example 1: Selection of ScFv binding to PD-1
[0131] Using an expanded version of the 1.38 x 10 10 The library's scFv phagemid library selects antibodies specific for human PD-1. Soluble PD-1 fusion protein (20 μg / ml in phosphate-buffered saline (PBS) solution) or control fusion protein (50 μg / ml in PBS solution) was coated into microtiter plate wells and left overnight at 4°C. Wells were washed in PBS and blocked in MPBS (3% milk powder in PBS) at 37°C for 1 hour. The purified phage (10 12Transducing units (tu) were blocked for 1 hour in 3% MPBS in a final volume of 100 μl. Add blocked phage to blocked control fusion protein wells and incubate for 1 hour. Blocked and deselected phage were then transferred to blocked wells coated with PD-1 fusion protein and incubated for an additional hour. Wells were washed 5 times with PBST (0.1% v / v Tween 20 in PBS) and then 5 times with PBS. Bound phage particles were eluted and used to infect 10 ml of exponentially growin...
Embodiment 2
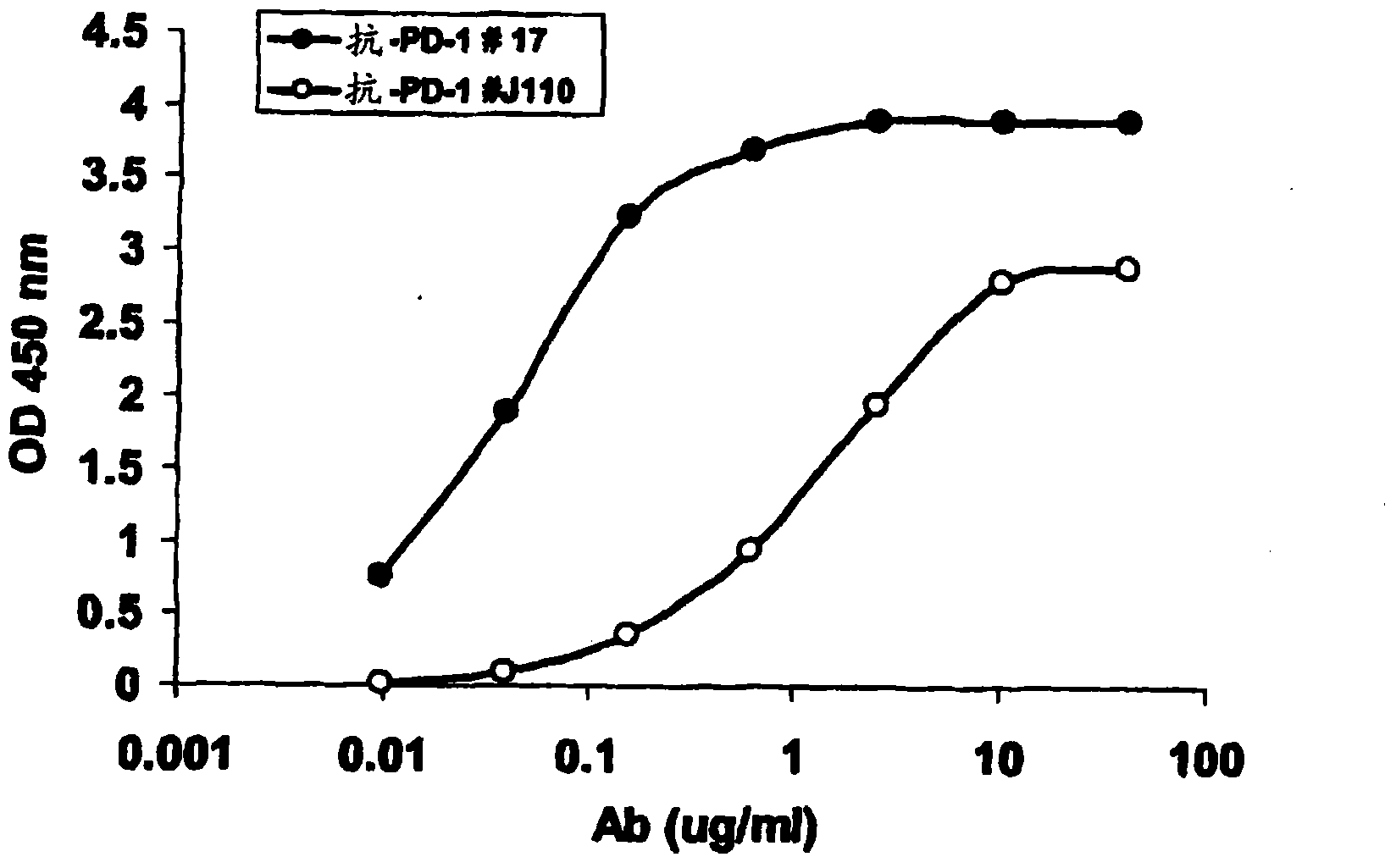
[0134] Example 2: Determination of antibody specificity to PD-1 by phage ELISA
[0135] To determine the specificity of the antibody against PD-1, phage ELISA was performed against PD-1 fusion protein and control protein. Individual E. coli colonies from selection products were picked into 96-well plates containing 100 [mu]l 2TYAG medium per well. M13K07 helper phage at a multiplicity of infection (moi) of 10 was added to the exponentially growing culture and the plate was incubated at 37°C for an additional 1 hour. The plate was centrifuged at 2000 rpm for 10 minutes in a table top centrifuge. The supernatant was removed and the cell pellet was resuspended in 100 μl 2TYAK and incubated overnight at 30°C with shaking. The next day, the plate was centrifuged at 2000 rpm for 10 minutes and the phage-containing supernatant from each well was transferred to a new 96-well plate. Phage samples were blocked in MPBS at a final concentration of 3% prior to ELISA.
[0136] 0.5-2.5...
Embodiment 3
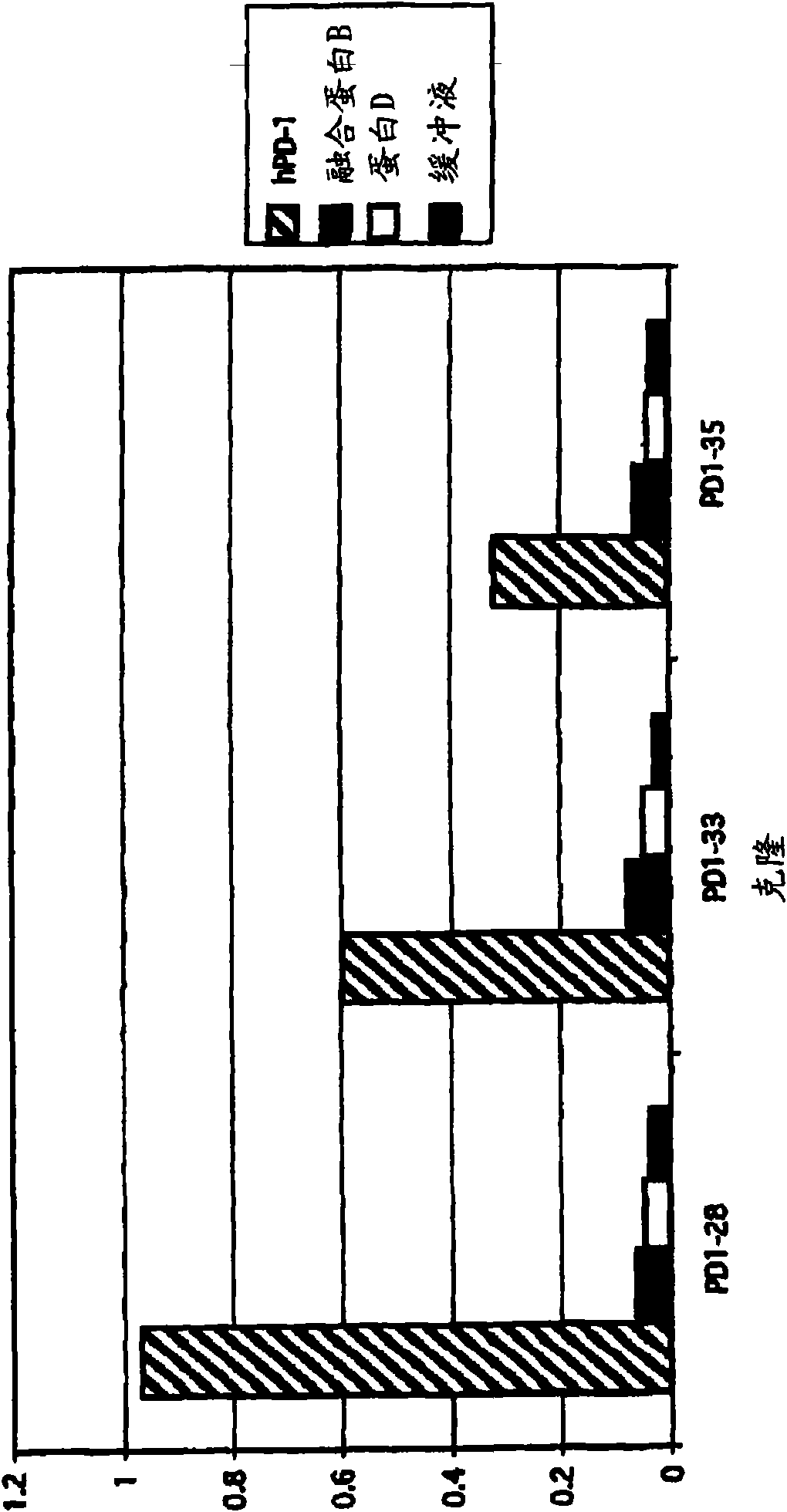
[0138] Example 3: Identification of antibody clones
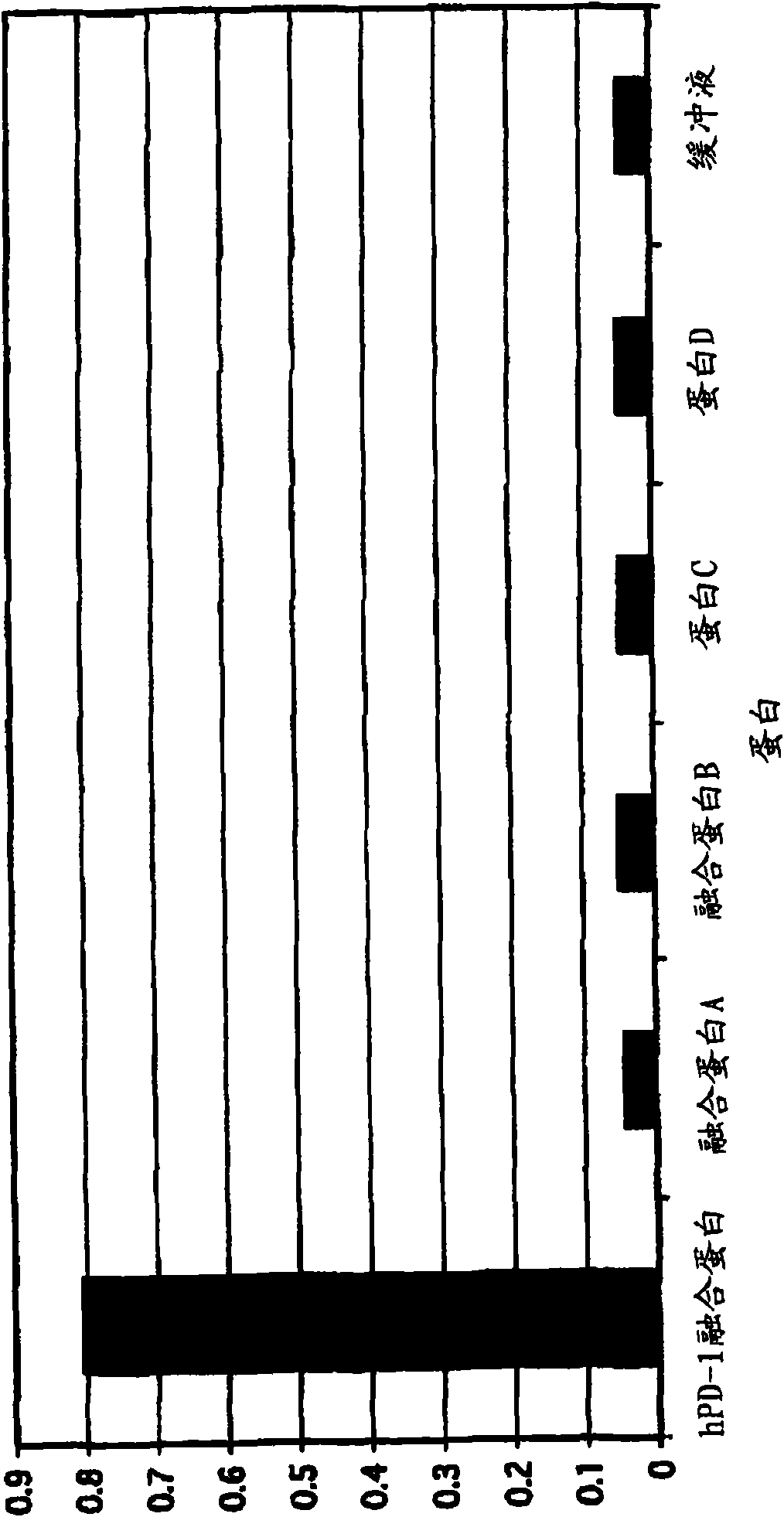
[0139] E. coli clones of PD-1 binding scFv were streaked onto 2TYAG plates and incubated overnight at 30°C. Amplification of V from scFv clones by using pCANTAB6 vector sequence oligonucleotides (oligos) H and V L Colonies from these plates were sequenced. Unique PD-1 binding clones that neutralized PD-L1 binding to PD-1 were assayed as described in Example 4. Sequence differences between scFv and IgG formats are due to changes introduced by PCR primers during conversion from scFv to IgG.
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com