Compositions and methods for delivery of glycopeptide antibiotics to medical device surfaces
A technology of peptide composition and medical device, applied in chemical instruments and methods, pharmaceutical formulations, medical science, etc., can solve problems such as urinary tract infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Phage display technology is commonly used to obtain peptides with binding affinities typically mediated by protein-peptide interactions. Phage display has also been successfully used to obtain peptides with binding affinity for surface materials such as metal or polymer surfaces. However, phage display has not been successfully used to obtain high binding affinity (e.g., by EC50 measured less than <1 mM). Thus, the present examples illustrate various methods of using phage display technology to unexpectedly obtain peptides with binding affinity (including high binding affinity) for glycopeptide antibiotics. Peptides with binding affinity for medical device surfaces were developed using first solid phase screening and phage display technology as previously described, followed by peptide design and peptide synthesis for improved binding properties.
[0048] In contrast to previously described phage selection methods, it was unexpectedly found during the development of t...
Embodiment 2
[0064] In this example, other features of the peptides according to the disclosed subject matter are illustrated.
[0065] Structure-function and binding affinity characterization
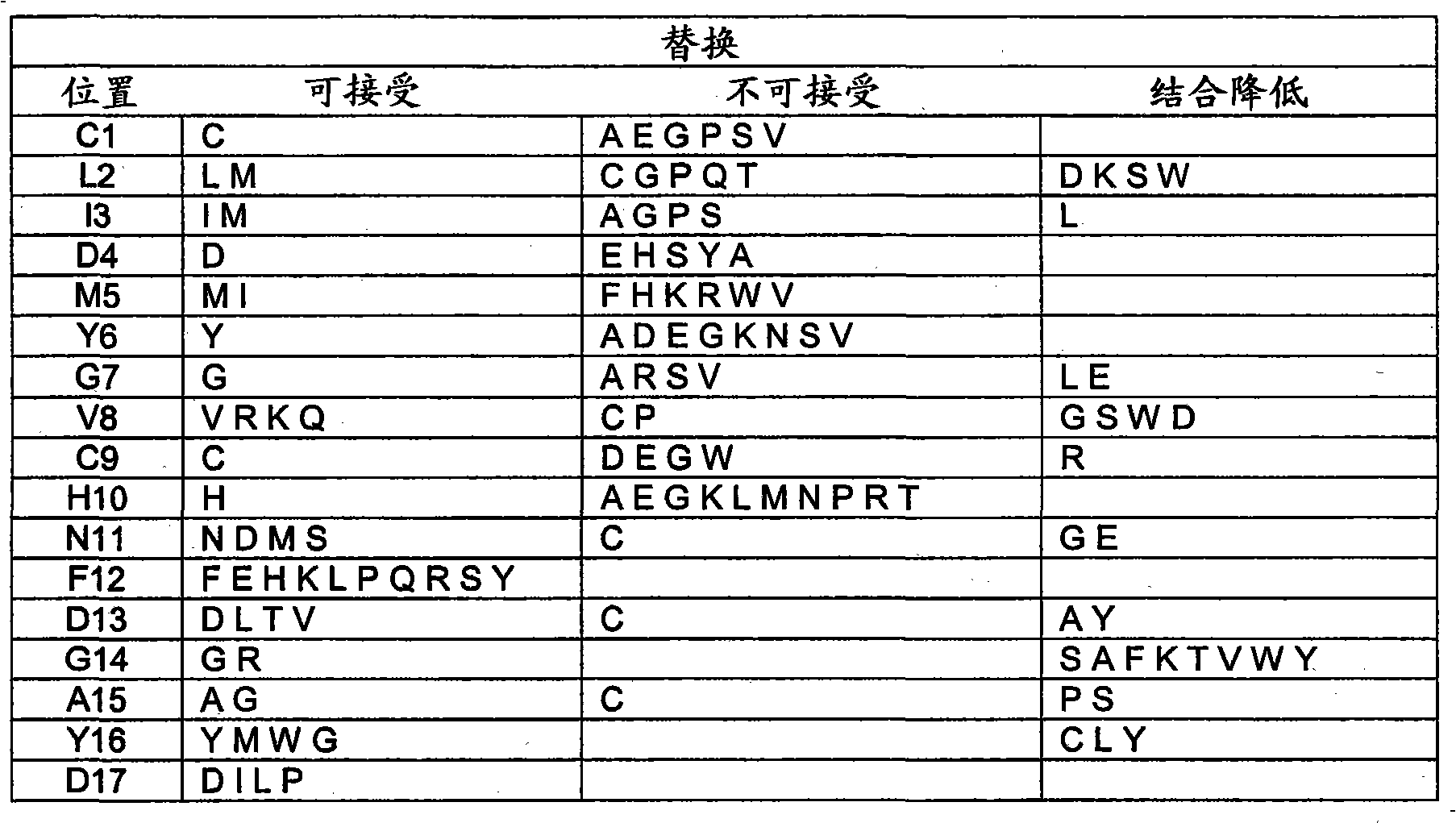
[0066] Additional peptides were designed starting from the peptide amino acid sequence SSCLIDMYGVCHNFDGAYDSSR (SEQ ID NO: 1) and expressed in the bacterial expression system described herein to further evaluate structure-function relationships. A truncated peptide comprising the amino acid sequence of SEQ ID NO:9 (STCLIDMYGVCH) was expressed and its binding affinity compared to a glycopeptide antibiotic comprising the peptide of SEQ ID NO:1. A dimer of this truncated peptide (SEQ ID NO: 10; STCLIDMYGVCHSSCLIDMYGVCH) with D replaced by A (SEQ ID NO: 11; STCLIAMYGVCH), D replaced by E (SEQ ID NO: 12; STCLIEMYGVCH) and G replaced by S substitution (SEQ ID NO: 13; STCLIDMYSVCH). The relative binding strength of these and other representative peptides to glycopeptide antibiotics was determined by th...
Embodiment 3
[0078] Although other surface-binding peptides known in the art may be used as components of compositions of the disclosed subject matter coupled to peptides of the disclosed subject matter having glycopeptide antibiotic binding affinity, Table 4 exemplifies the Representative peptides for the binding affinity of the material ("surface-bound peptides"). For example, the surface-binding peptide comprises the following amino acid sequences: SEQ ID NO: 14-35 with binding affinity for polystyrene; SEQ ID NO: 36 with binding affinity for polyurethane; SEQ ID NOs: 37-50 with polycarbonate binding affinity; SEQ ID NOs: 51-56 with polycarbonate binding affinity; SEQ ID NOs: 57-65 with nylon binding affinity; with Teflon binding affinity SEQ ID NOS: 66 and 67; SEQ ID NOS: 68 and 69 with binding affinity for polyethylene terephthalate fibers; SEQ ID NOS: 70 and 71 with binding affinity for collagen-based substrates; SEQ ID NOS: 72-119 having binding affinity for metals (eg, including o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com