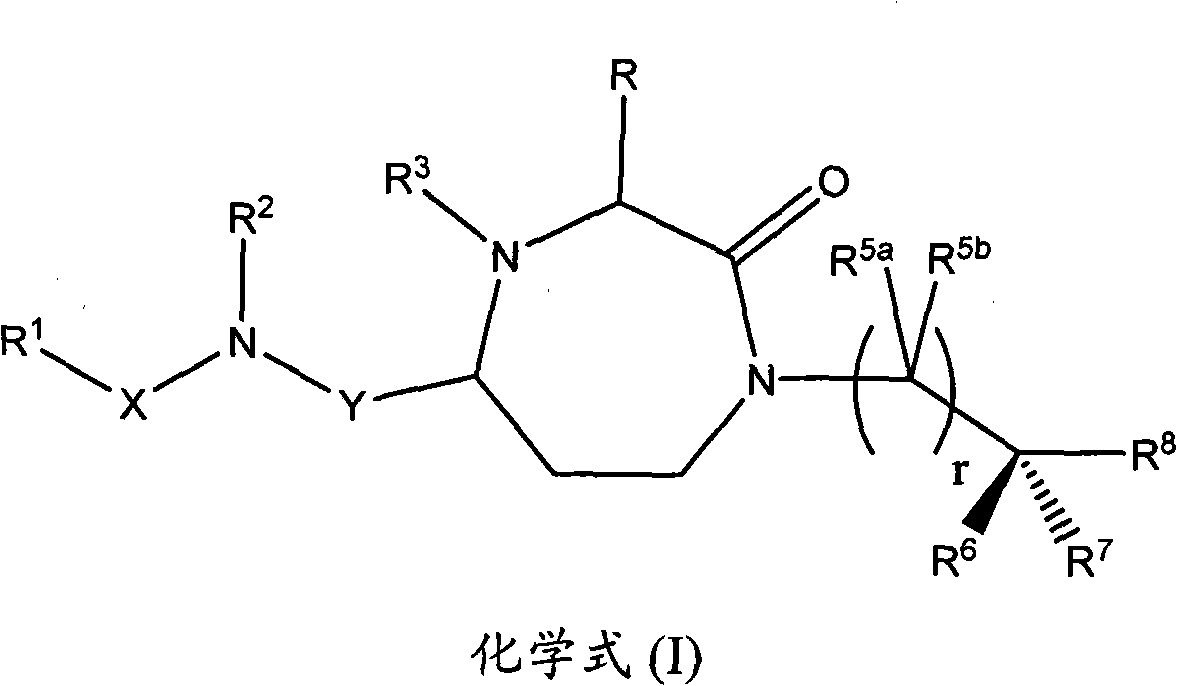
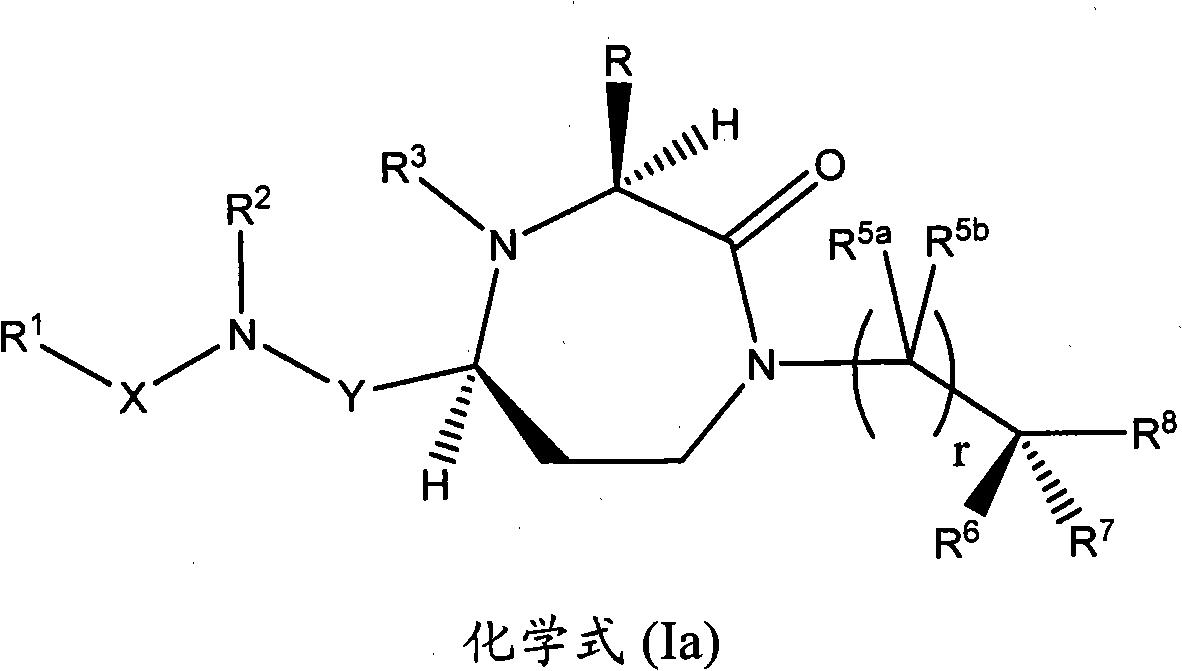
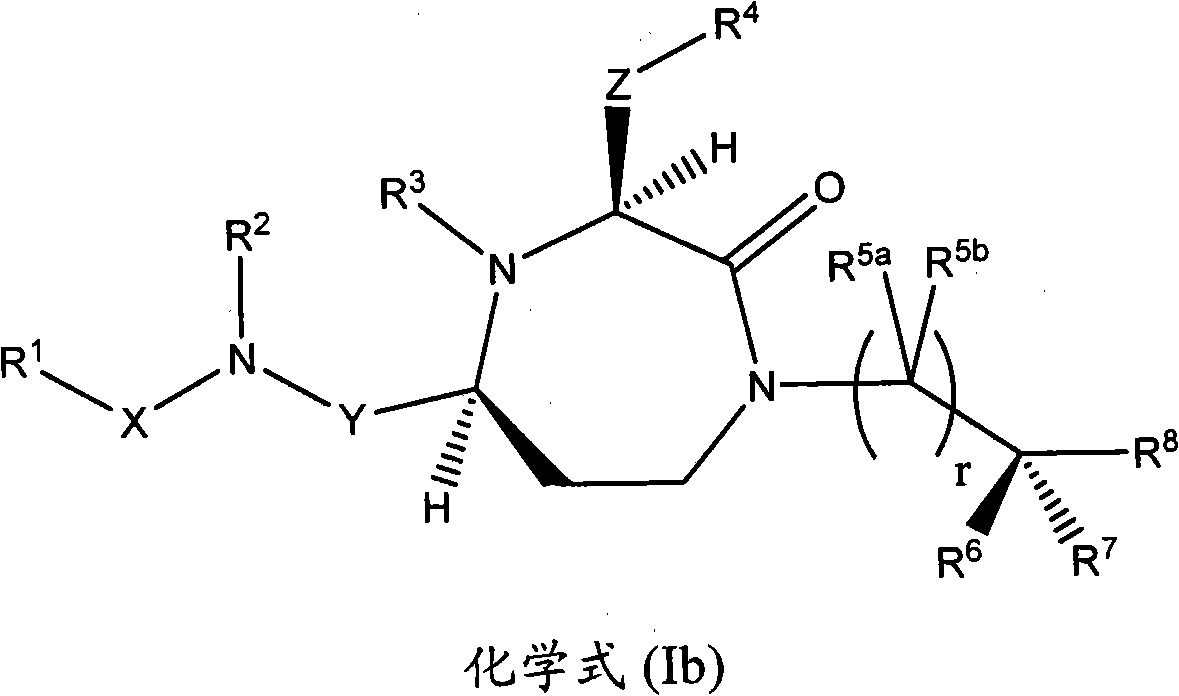
Methods of modulating the activity of the mc5 receptor and treatment of conditions related to this receptor
A stereochemical, C2-C12 technology, applied in the direction of organic active ingredients, skin diseases, pharmaceutical formulations, etc., can solve problems such as side effects and seriousness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0833] The following examples are intended to illustrate the disclosed embodiments and should not be construed as limitations thereon. In addition to those illustrated below, additional compounds can be prepared using the reaction schemes illustrated below (as discussed above) or appropriate variations or variations thereof. All starting materials illustrated in the following examples are either commercially available or readily synthesized by one of ordinary skill in the art.
[0834] Equipment
[0835] HPLC analysis was performed on an Agilent 1100 series purification system equipped with a Phenomenex Synergi 4μ Max-RP 80A, 50x2.00mm analytical HPLC column with peak detection by UV light. The standard assay employed 0.05% trifluoroacetic acid (TFA) in water (solvent A) and 0.05% TFA in 90:10 acetonitrile:water (solvent B) at a flow rate of 1 mL / min over 9 minutes. A gradient of 5% B (initial) to 95% B was used. Mass spectra were performed on an Applied Biosystems MDS Scie...
example 1
[0837] Example 1 - General Operations - Formation of Weinreb Amides
[0838]
[0839] BOP reagent (100 mmol) and diisopropylethylamine (DIPEA) (100 mmol) were added to a stirred solution of amino acid (1) (100 mmol) in dichloromethane (DCM) (100 mL). The solution was then stirred at room temperature for 10 minutes before adding a premixed solution of N,O-dimethylhydroxylamine hydrochloride (100 mmol) and DIPEA (100 mmol), followed by overnight stirring at room temperature. Then, DCM was removed by rotary evaporation and the residue was taken up in ethyl acetate (EtOAc) (200 mL). Then, the organic phase was washed with 1N HCl (3x100 mL), H 2 O (3x100mL), saturated NaHCO 3 Aqueous solution (3x100 mL) and brine (1x10 mL) were washed. Then, the organic phase was dried (MgSO 4 ) and EtOAc was removed to give Weinreb amide (2) as a white solid or an oil.
example 2
[0840] Example 2 - General operation - Vinyl Grignard addition to Weinreb amides to form β-unsaturated ketones
[0841]
[0842] To Weinreb amide (2) (15 mmol) in DCM (10 mL) at 0°C was added vinylmagnesium bromide (45 mmol) in THF (45 mL). The reaction was stirred for 2 hours and monitored by HPLC. The reaction was then quenched by adding the reaction to a mixture of ice and 1M HCl (200 mL). The aqueous mixture was extracted with DCM (3x100 mL), and the organic layers were combined and washed with 1M HCl (2x200 mL) and H 2 O (3x100 mL) was used for washing. The organic phase was dried (MgSO 4 ) to provide Solutions of β-unsaturated ketones (3). The β-unsaturated ketone (3) can be isolated by rotary evaporation, or it can be used in solution without further purification. If the purpose is to use in solution β-Unsaturated ketone (3), the volume was reduced to 100 mL by rotary evaporation and saved for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com