Enzymatic analytical membrane, test device and method
A technology for enzyme analysis, analyte, applied in measurement devices, biochemical equipment and methods, enzymology/microbiology devices, etc., which can solve problems such as confusion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1: Evaluation of Enzyme Assays for the Detection of Acetaminophen (APAP) and Methanol (MeOH) membrane
[0082] Twelve sera from two different MeOH overdoses and 17 sera positive for APAP by immunoassay were analyzed by the enzymatic assay membrane described herein. In addition to commercially available quality control samples, 6 and 10 sera that were negative for MeOH and APAP, respectively, were also analyzed. Cross-reactivity to ethanol was tested with 40 mmol, 80 mmol and 120 mmol of ethanol. Inspection technicians ignore initial results.
[0083] MeOH: Nine out of eleven POCT positive samples were positive according to the results of gas chromatography (GC). The results are read at two minutes. The two samples that were positive by the enzyme assay membrane and negative by GC were only below the detection limit of the GC, suggesting that the enzyme assay membrane may be more sensitive than the GC. The detection limit of GC is about 2 mM. Consistent ...
Embodiment 2
[0086] Example 2: Membrane device for acetaminophenase analysis
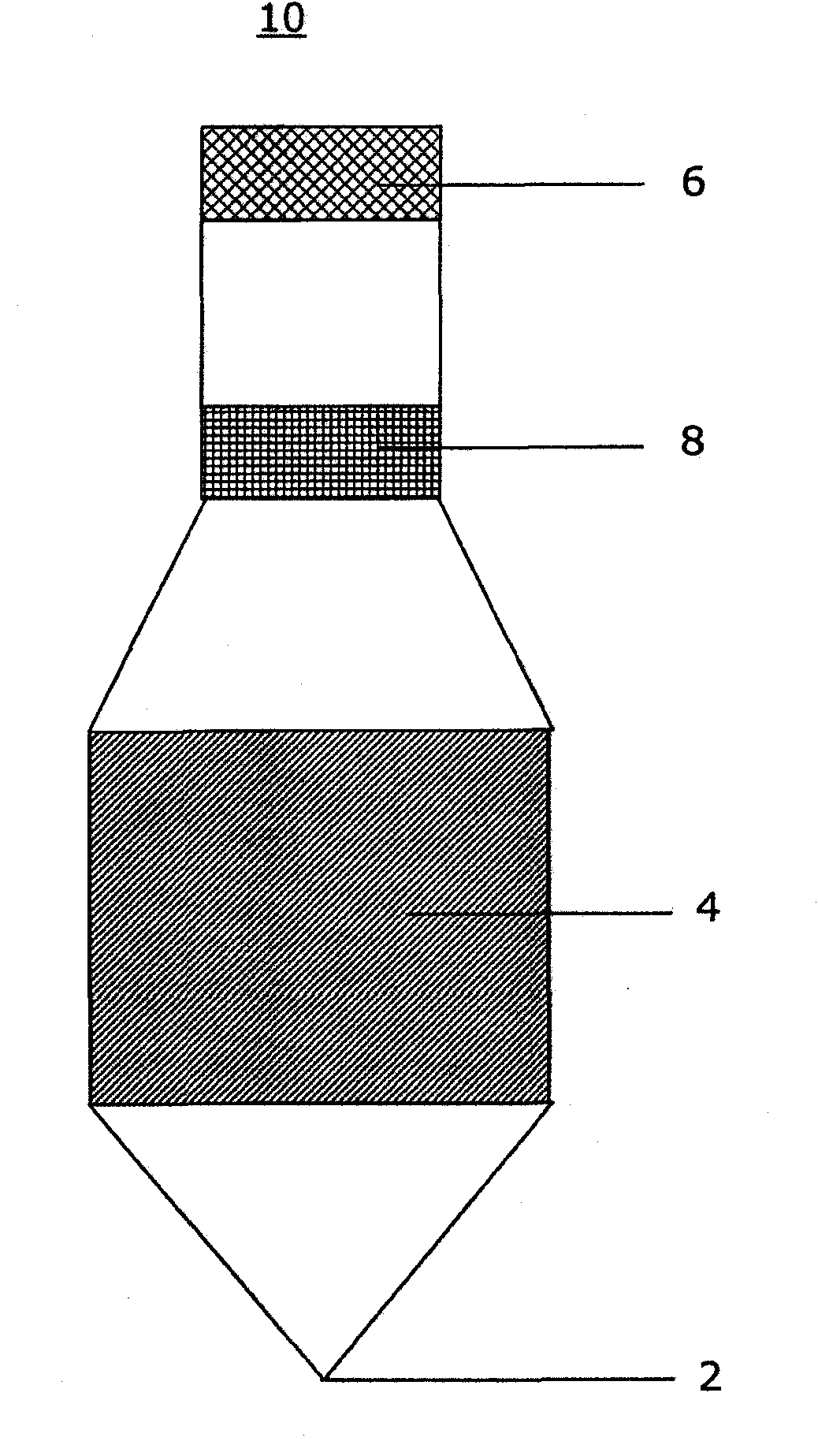
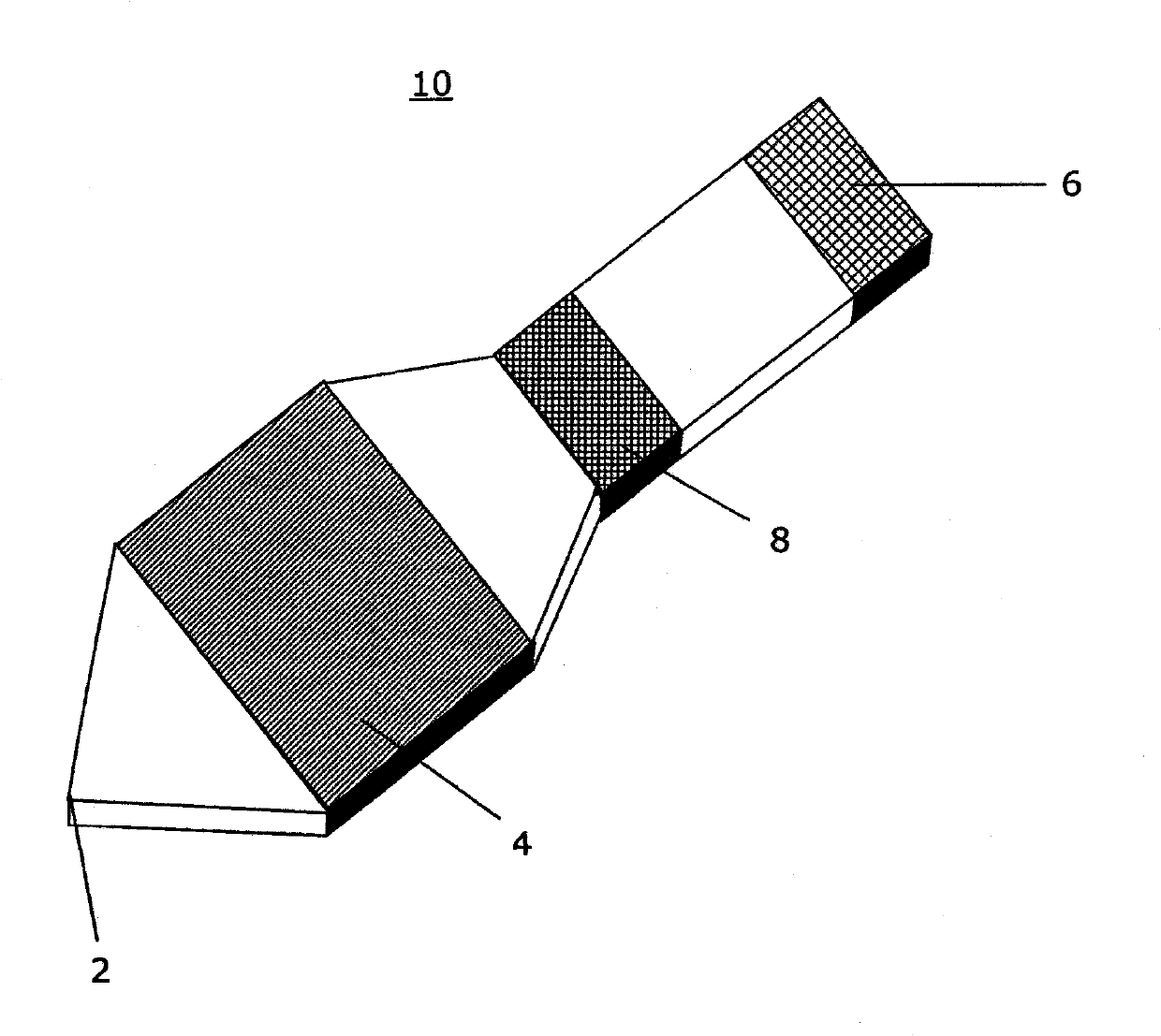
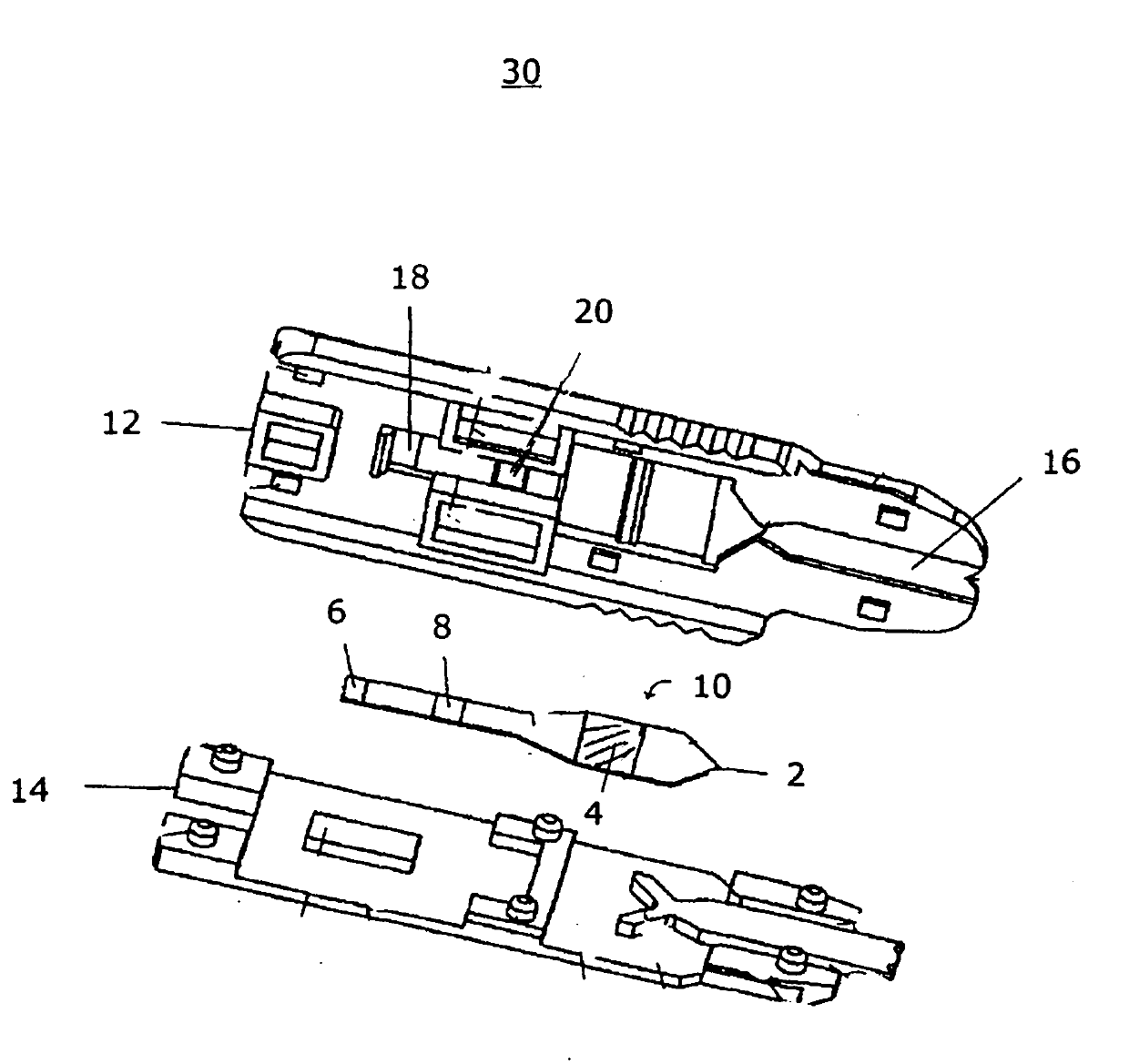
[0087] An acetaminophen detection device using a drop of whole blood sample was prepared according to the present invention. For the signal area, nitrocellulose (Millipore) was infiltrated with the chromogenic reagent at a specified flow rate of 180 sec / 4 cm using a conventional liquid sprayer. Chromogenic reagents included 3% o-cresol, 6.4 mM copper sulfate and 1M sodium hydroxide. The infiltrated nitrocellulose was left for 30 minutes at room temperature at less than 20% relative humidity. The separation zone (Whatman) was sprayed with enzyme solution and then lyophilized to remove water. The enzyme solution included 250 U / mL aryl acyl amidase (GDS Technology). Enzyme assay membranes were supported on polystyrene liners (G & L Precision Die Cutting, Inc.). Use a die-cut tool to obtain the shape of the enzymatic assay membrane. Such as image 3 As shown, place the enzyme analysis membrane in the analysis ...
Embodiment 3
[0088] Example 3: Membrane device for methanolase analysis
[0089] A methanol detection device using a drop of whole blood sample was prepared according to the present invention. For the signal area, infiltrate nitrocellulose at a prescribed flow rate of 180 sec / 4 cm with chromogenic reagent, 18 U / mL formaldehyde dehydrogenase (Sigma-Aldrich) and 6 mM β-nicotinamide adenine dinucleotide hydrate (β-NAD) ( Millipore). Chromogenic reagents included 0.3 mM phenazine methyl sulfate, 1.2 mM nitro blue tetrazolium chloride and 0.5% Triton X-100. The separation zone (Whatman) was sprayed with 315 U / mL alcohol oxidase solution (Sigma-Aldrich). The soaked membranes were left for 30 minutes at room temperature at a relative humidity below 20%. Enzyme assay membranes were supported on polystyrene liners (G & L Precision Die Cutting, Inc.). Use a die-cut tool to obtain the shape of the enzymatic assay membrane. Such as image 3 As shown, place the enzyme analysis membrane in the a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com