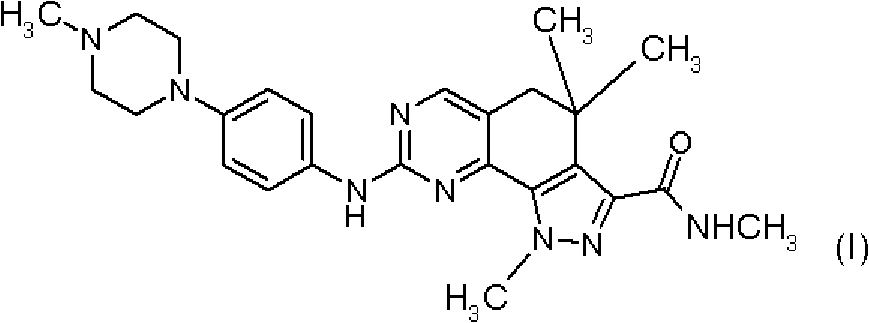
Therapeutic combination comprising a cdks inhibitor and an antineoplastic agent
An anti-tumor agent and inhibitor technology, which can be used in anti-tumor drugs, drug combinations, medical preparations containing active ingredients, etc., and can solve problems such as increased cancer incidence.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
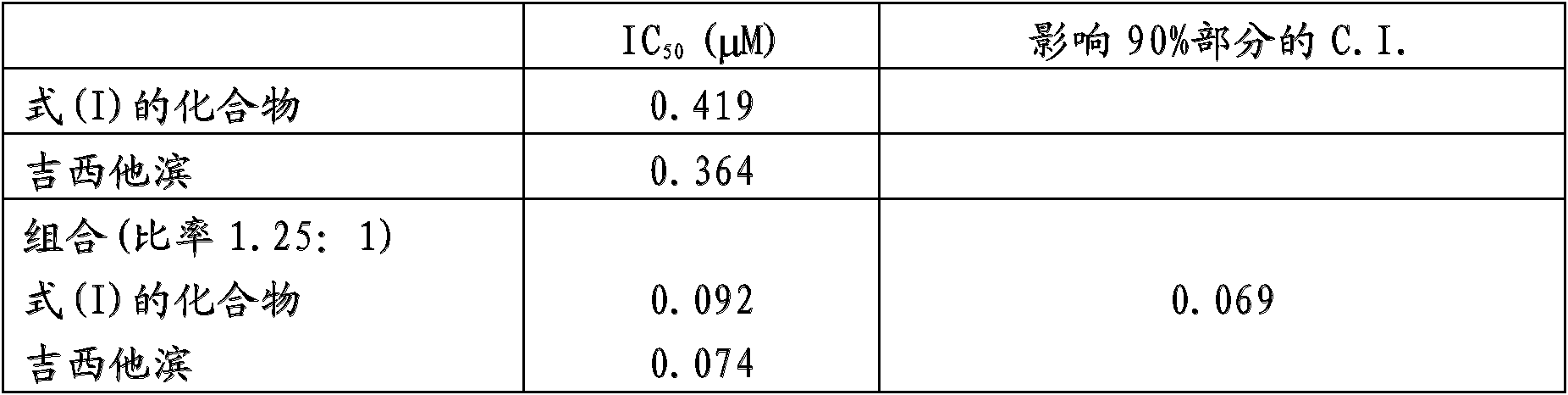
[0046] Example 1: In vitro cytotoxic activity of combinations with gemcitabine
[0047] Implant the exponentially growing A2780 human ovarian cancer cell line and incubate at 37°C in a humidified 5% CO 2 Cultivated under atmosphere. After 24 hours, a scalar dose of a compound of formula (I) as defined above is added to the medium for 24 hours, the cells are washed and gemcitabine is added for 1 hour. Cells were then washed and counted 72 hours after the first treatment. Cell proliferation was measured by an intracellular ATP monitoring system. Cell proliferation was compared to control cells. Calculate the concentration that inhibits 50% cell proliferation (IC 50 ).
[0048] The Combination Index (C.I.) is calculated using a computer program for the analysis of effects of multiple drugs based on the Chou-Talalay equation for mutually non-exclusive drugs (Adv Enzyme Regul 1984; 22:27-55), where C.I. and effects.
[0049] The results obtained with the drugs alone and in c...
Embodiment 2
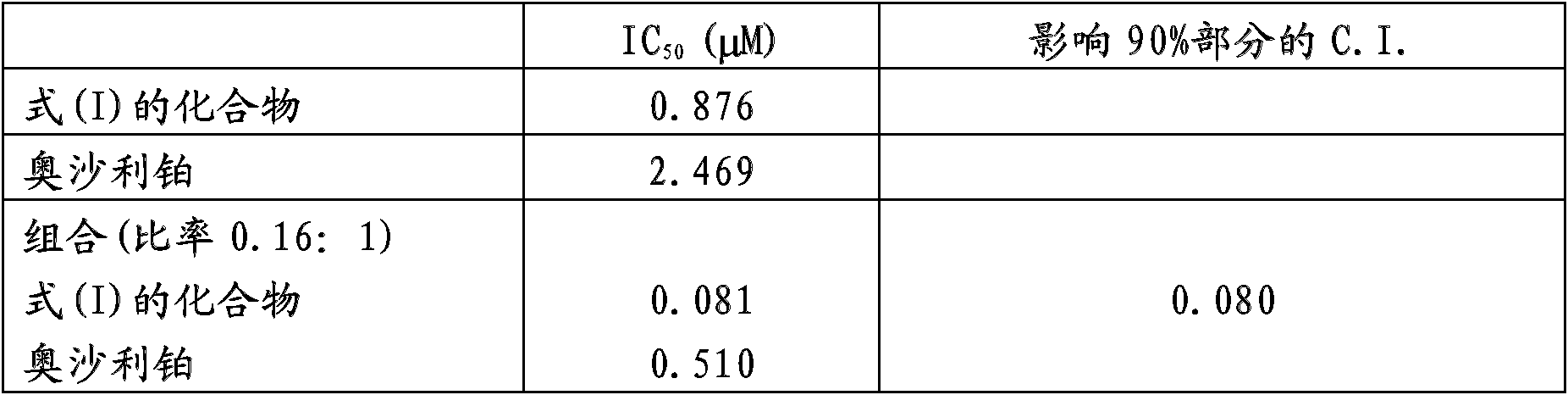
[0053] Example 2: In vitro cytotoxic activity of combination with oxaliplatin
[0054] Implant the exponentially growing A2780 human ovarian cancer cell line and incubate at 37°C in a humidified 5% CO 2 Cultivated under atmosphere. After 24 hours, a scalar dose of oxaliplatin is added to the medium for 1 hour, the cells are washed and the compound of formula (I) is added for 24 hours. Cells were then washed and counted 72 hours after the first treatment. Cell proliferation was measured by an intracellular ATP monitoring system. Cell proliferation was compared to control cells. Calculate the concentration that inhibits 50% cell proliferation (IC 50 ).
[0055] The Combination Index (C.I.) is calculated using a computer program for the analysis of effects of multiple drugs based on the Chou-Talalay equation for mutually non-exclusive drugs (Adv Enzyme Regul 1984; 22:27-55), where C.I. and effects.
[0056] The results obtained with the drugs alone and in combination are s...
Embodiment 3
[0060] Example 3: In vitro cytotoxic activity of combinations with SN-38
[0061] SN38 is the active metabolite of irinotecan, which is obtained by hydrolysis of irinotecan. Implant the exponentially growing A2780 human ovarian cancer cell line and incubate at 37°C in a humidified 5% CO 2 Cultivated under atmosphere. After 24 hours, a scalar dose of SN-38 is added to the medium for 1 hour, the cells are washed and the compound of formula (I) is added for 24 hours. Cells were then washed and counted 72 hours after the first treatment. Cell proliferation was measured by an intracellular ATP monitoring system. Cell proliferation was compared to control cells. Calculate the concentration that inhibits 50% cell proliferation (IC 50 ).
[0062] The Combination Index (C.I.) is calculated using a computer program for the analysis of effects of multiple drugs based on the Chou-Talalay equation for mutually non-exclusive drugs (Adv Enzyme Regul 1984; 22:27-55), where C.I. and eff...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com