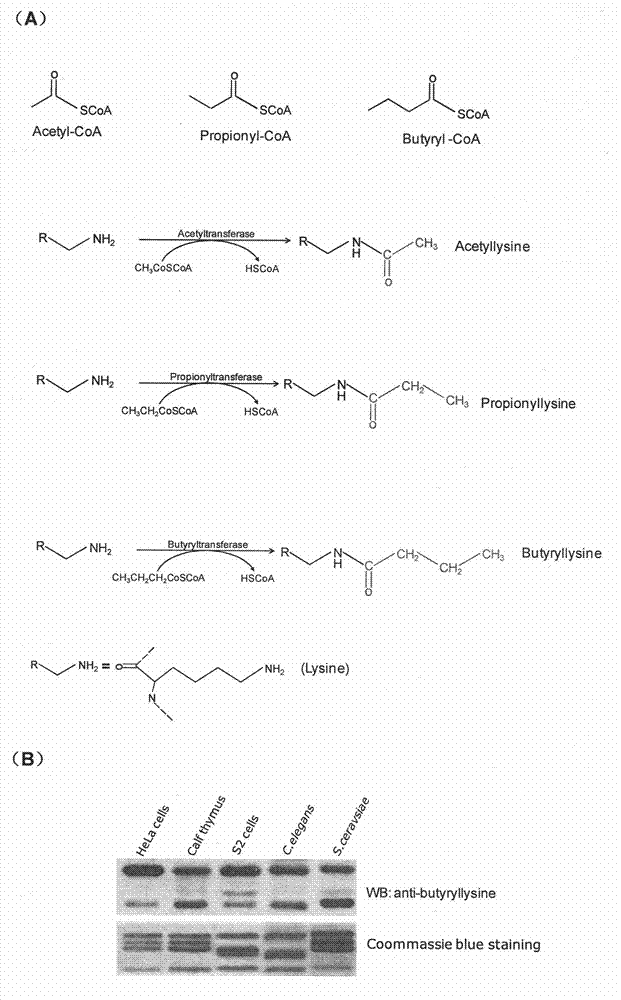
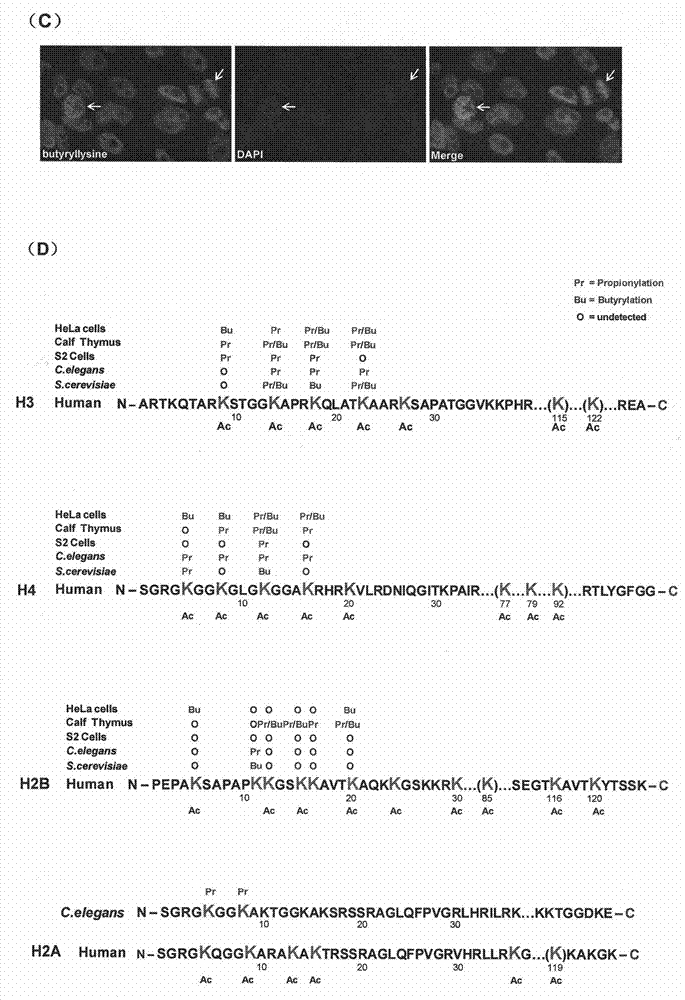
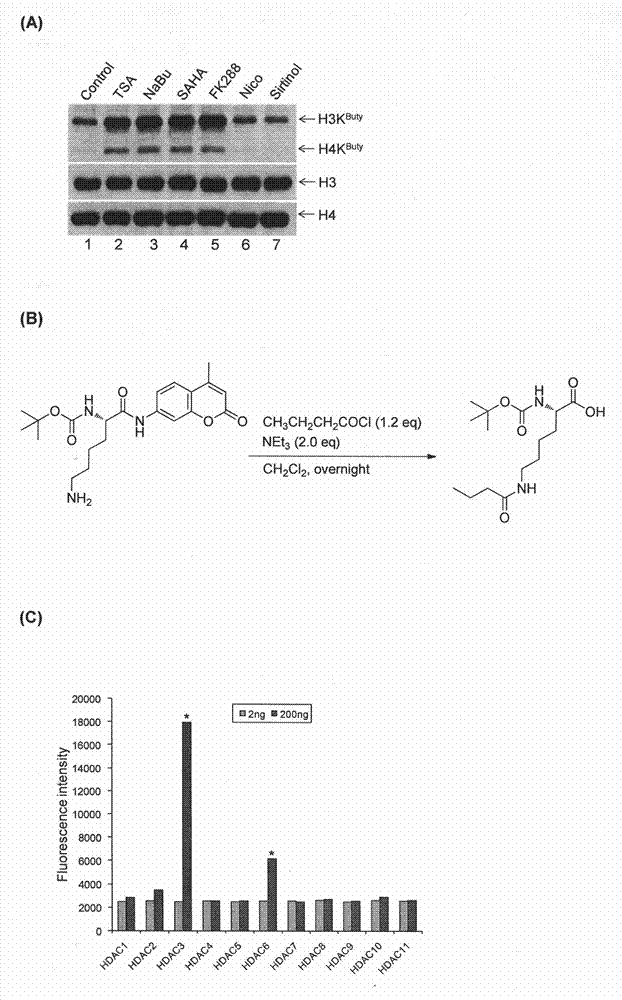
Method for screening and testing activity of lysine propionylation removal enzyme and butyrylation removal enzyme
A technology for removing butyrylase and propionylase, which can be used in the determination/inspection of microorganisms, biochemical equipment and methods, measuring devices, etc., and can solve problems such as research and affecting biological functions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] Plasmids, Antibodies and Other Reagents Plasmids encoding HDAC3, HDAC6 cDNA and their corresponding enzyme-inactive mutant plasmids HDAC3S424A and HDAC6H216 / 611A were kindly provided by Edward Seto, H. Lee Moffitt Cancer Center. See previous reports for plasmid information (21-22) . The anti-butyryl-lysine antibody and the anti-histone site-specific butyryl-lysine antibody were prepared by the inventors themselves. Other antibodies used were: anti-acetylated lysine antibody (ImmunoChem Pharmaceuticals, Burnaby, British Columbia, Canada); anti-histone H3 antibody, anti-histone H4 antibody (Abcam, Cambridge, UK); anti-HA antibody (Roche Diagnostics , Indianapolis, IN); anti-Flag antibody (Sigma-Aldrich, St. Louis, MO). Recombinant HDAC1, HDAC2, HDAC3, HDAC4, HDAC5, HDAC6, HDAC7, HDAC8, HDAC9, HDAC10 and HDAC11 were purchased from BPS Bioscience Inc. (BPS Bioscience Inc., San Diego, CA). Butyryl-CoA, acetyl-CoA, Leptomycin B and acetate were purchased from Sigma-Aldrich...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com