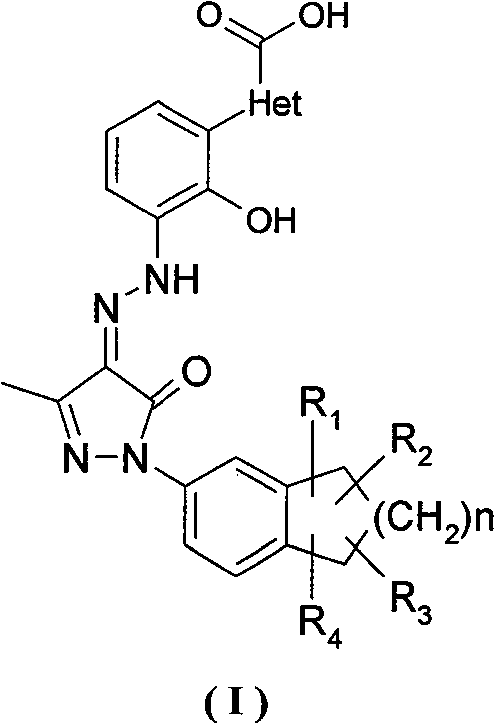
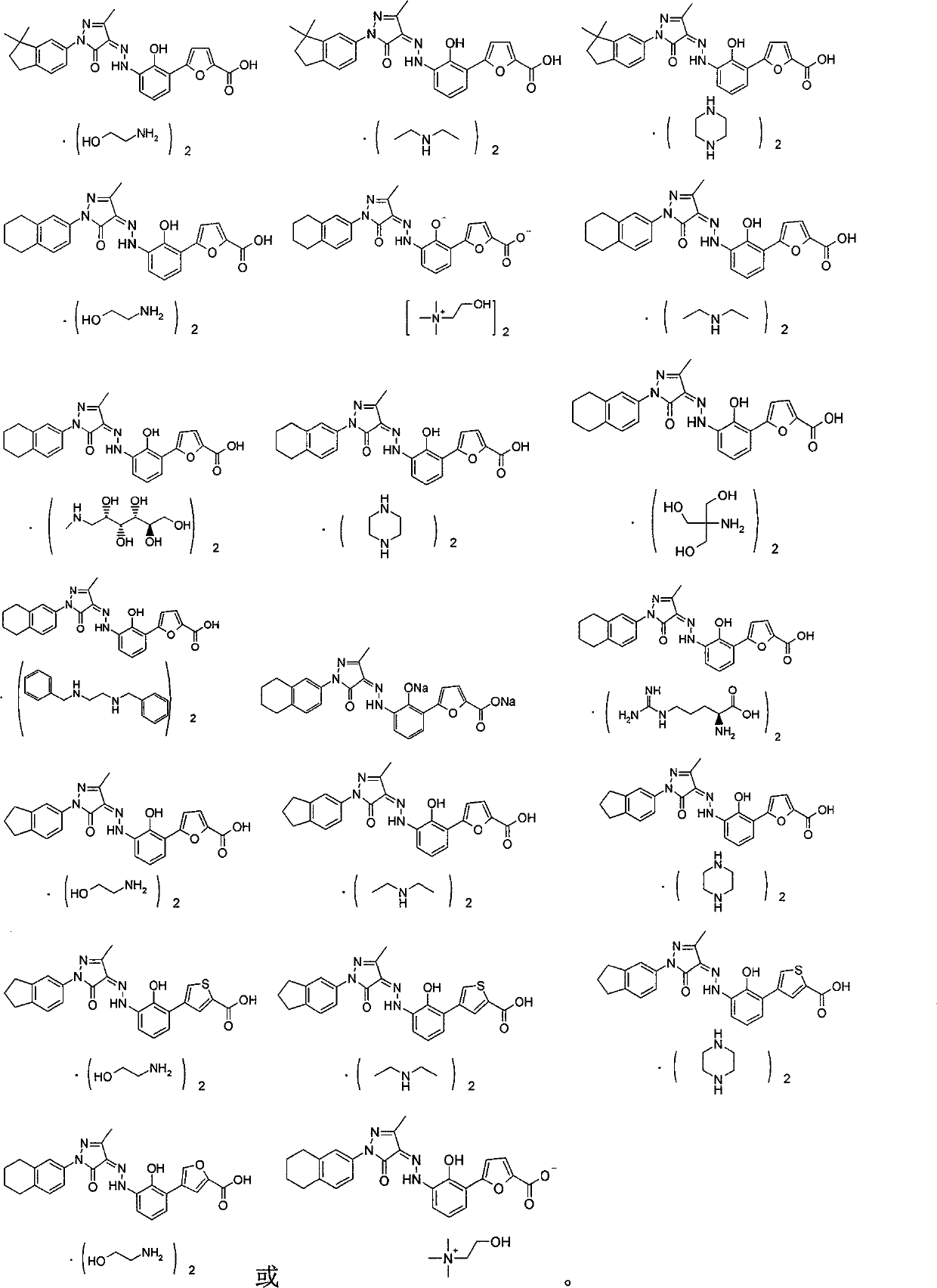Salts of bicyclo-substituted pyrazolon azo derivatives, preparation method and use thereof
A drug and compound technology, applied in the field of thrombopoietin mimics and thrombopoietin receptor agonists, can solve the problems of not specifying the form of the salt, reducing the bioavailability of the compound in vivo, and poor solubility of the compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
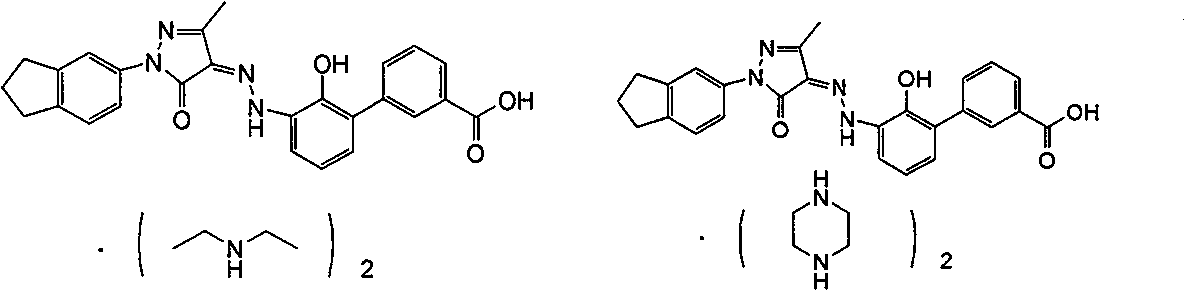
[0113] (Z)-2'-Hydroxy-3'-[N'-(1-indan-5-yl-3-methyl-5-oxo-1,5-dihydropyrazole-4-ylidene) -hydrazino]-biphenyl -3-Carboxylic acid diethanolamine salt
[0114]
[0115]
[0116] first step
[0117] 2-Bromo-6-nitrophenol
[0118] Dilute 60mL of concentrated sulfuric acid into 186mL of water, add sodium nitrate (79.2g, 0.93mol) after cooling to room temperature, keep below 25°C, add o-bromophenol 1a (60mL, 0.52mol) dropwise, and react at room temperature for 2 hours. Add 320mL ethyl acetate to dissolve the separated solid, wash with water and saturated brine respectively, dry over anhydrous magnesium sulfate, filter, concentrate the filtrate under reduced pressure, and purify the resulting residue by silica gel column chromatography to obtain 2-bromo-6-nitrate phenylphenol 1b (48.2 g, yellow solid). Yield: 42.8%.
[0119] MS m / z(ESI): 218[M+1]
[0120] 1 H NMR (400MHz, CDCl 3 ): δ11.18(s, 1H), 8.12-8.15(m, 1H), 7.89-7.91(m, 1H), 6.88-7.02(m, 1H)
[0121] second s...
Embodiment 2
[0160] (Z)-2'-Hydroxy-3'-[N'-(1-indan-5-yl-3-methyl-5-oxo-1,5-dihydropyrazole-4-ylidene) -hydrazino]-biphenyl -2-Carboxylic acid bis(diethylamine) salt
[0161]
[0162] (Z)-2'-hydroxyl-3'-[N'-(1-indan-5-yl-3-methyl-5-oxo-1,5-dihydropyrazole-4-ylidene )-hydrazino]-biphenyl-3-carboxylic acid 1j (150mg, 0.33mmol) was dissolved in 5mL tetrahydrofuran, and it was a dark red suspension. Diethylamine (48mg, 0.66mmol) was added dropwise under stirring, and it was purple Red solution, reacted at room temperature for 2 hours. A solid precipitated in the reaction solution, filtered, the filter cake was washed with tetrahydrofuran (1mL×3), and the obtained solid was dried under vacuum to obtain the title product (Z)-2′-hydroxyl-3′-[N′-(1- Indan-5-yl-3-methyl-5-oxo-1,5-dihydropyrazol-4-ylidene)-hydrazino]-biphenyl-3-carboxylic acid bis(diethylamine) Salt 2 (132 mg, red solid), yield: 66.7%.
[0163] HPLC: 99.2%
[0164] MS m / z(ESI): 452.9[M-1]
[0165] 1 H NMR (400MHz, CD 3...
Embodiment 3
[0167] (Z)-2'-Hydroxy-3'-[N'-(1-indan-5-yl-3-methyl-5-oxo-1,5-dihydropyrazole-4-ylidene) -hydrazino]-biphenyl -3-Carboxylic acid dipiperazine salt
[0168]
[0169] (Z)-2'-hydroxyl-3'-[N'-(1-indan-5-yl-3-methyl-5-oxo-1,5-dihydropyrazole-4-ylidene )-hydrazino]-biphenyl-3-carboxylic acid 1j (150mg, 0.33mmol) was dissolved in 5mL tetrahydrofuran, and it was a dark red suspension, and piperazine (57mg, 0.66mmol) was added under stirring, and it was a purple-red solution , stirred at room temperature for 2 hours. A solid precipitated in the reaction solution, filtered, the filter cake was washed with tetrahydrofuran (1mL×3), and the obtained solid was dried under vacuum to obtain the title product (Z)-2′-hydroxyl-3′-[N′-(1- Indan-5-yl-3-methyl-5-oxo-1,5-dihydropyrazol-4-ylidene)-hydrazino]-biphenyl-3-carboxylic acid dipiperazine salt 3( 130 mg, dark red solid), yield: 62.8%.
[0170] HPLC: 98.5%
[0171] MS m / z(ESI): 452.8[M-1]
[0172] 1 H NMR (400MHz, CD 3OD): δ8.1...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap