globo H and related anticancer vaccines containing novel glycolipid adjuvants
A glycan and antigen technology, applied in the field of anti-cancer GloboH-DT vaccine, can solve the problem of difficulty in the precise determination of glycocomplexation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0182] Example 1: Synthesis of GloboH complexed with different carrier proteins
[0183] GloboH was synthesized using a programmable one-pot strategy for process control (1; see Figure 11) and its fragment 2-10 (Huang C-Y, et al. (2006) ProcNatlAcadSciUSA103: 15-20). The reaction of 1 is carried out with sufficient homobifunctional (homobifunctional) linkers in anhydrous DMF solution at room temperature (WuX, et al. (2004) Org Lett6: 4407-4410; WuX, BundleDR (2005) JOrgChem70 :7381-7388). The reaction can be easily monitored by TLC. Once the free amine disappeared and the larger Rf product appeared, the reaction mixture was evaporated to remove DMF and washed with dichloromethane and water to remove excess linker. Finally, the product was purified by reverse-phase (C18) column chromatography and gradually eluted with water containing 1% acetic acid to 40% methanol in water. The solution was then lyophilized to yield the pale yellow product 12. Finally, for protein comple...
example 2
[0186] Example 2: Fabrication and Validation of Glycan Microarrays
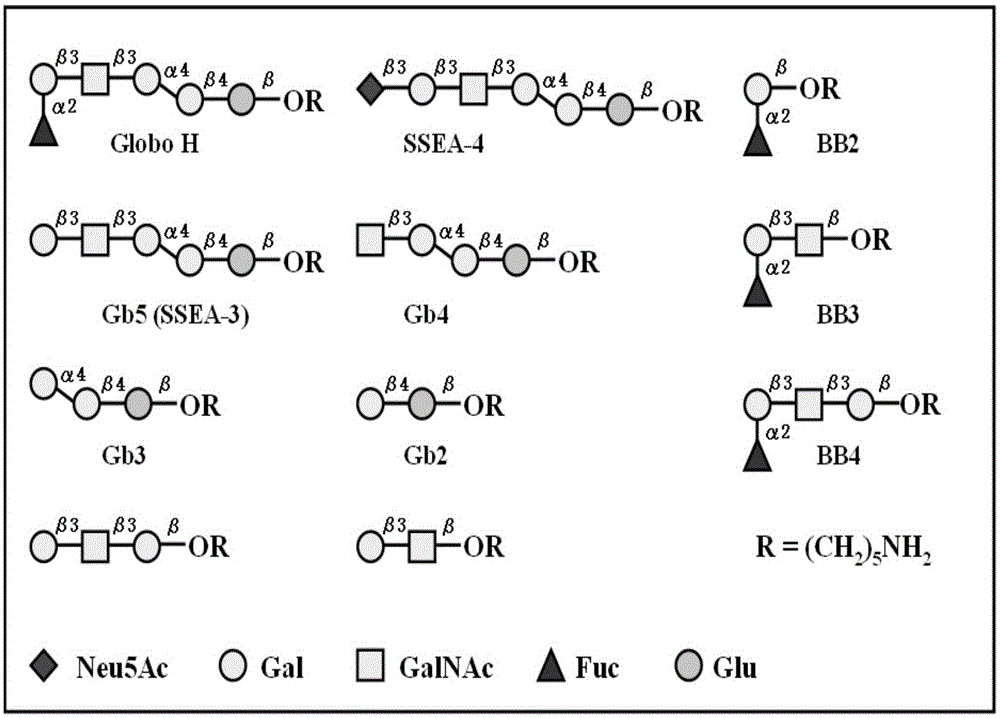
[0187] Make the synthetic GloboH and cut fragment ( figure 1 ) was attached to the reducing end with a pentylamine linker and covalently immobilized on NHS-coated glass slides. Of the eleven oligosaccharides, nine were selected to be imprinted on the microarray. A series of oligosaccharide concentrations (1, 5, 10, 20, 40, 50, 80, 100 [mu]M) were tested to optimize binding affinity and fluorescence intensity. Each microarray slide was spotted with 12 replicates of nine GloboH analogs (SSEA-4, GH, Gb5, Gb4, Gb3, Gb2, BB4, BB3, and BB2) at 50 μM each. After reacting in an 80% humidity atmosphere, the slides were stored in a desiccator at room temperature until use.
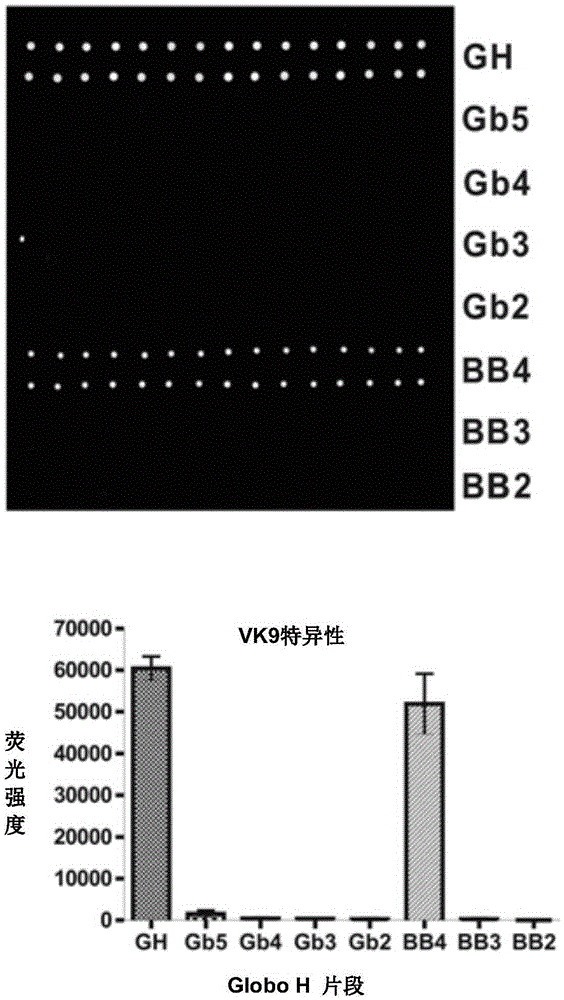
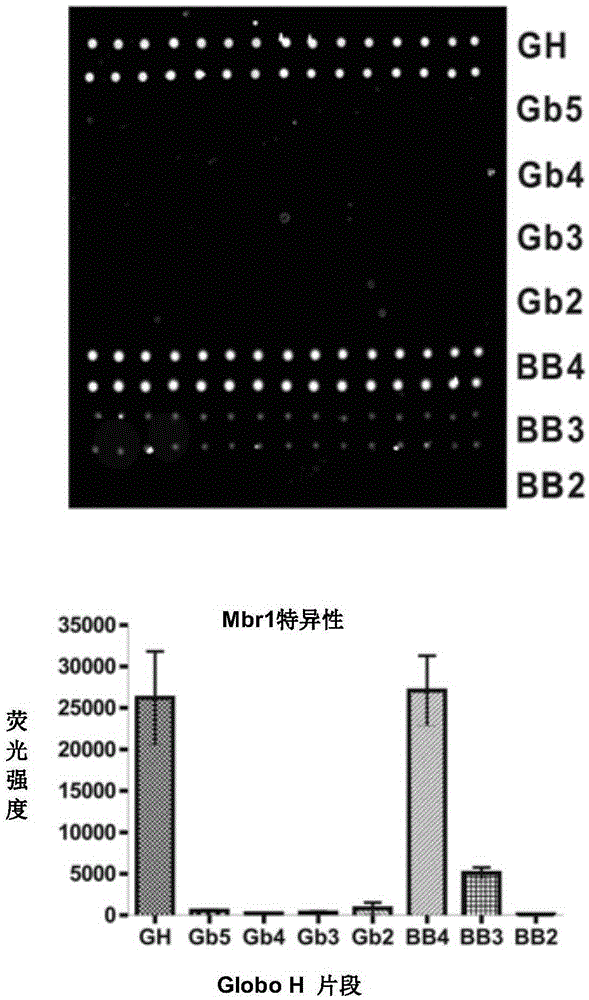
[0188] To validate carbohydrates on microarrays, mouse monoclonal antibodies (VK9 and Mbr1 against GloboH, and anti-SSEA-3) and corresponding secondary antibodies (goat anti-mouse IgG and IgM) were used to test pool specificity , and the result...
example 3
[0189] Example 3: Mouse Immunization
[0190] In this experiment, a group of mice were immunized subcutaneously with 1 μg of the synthetic GloboH(GH)-complex in the presence or absence of the glycolipid adjuvant α-GalCer(C1). After 10 days of three immunizations at weekly intervals, mouse sera were collected and subsequently introduced into glycan microarrays to assess antibody levels. It is found that GH-KLH, GH-DT, and GH-BV are the most effective immunogens induced by IgM, followed by GH-TT and GH-BSA, such as Figure 3A Abstract, and α-GalCer can stimulate the immune response to induce high levels of IgM antibodies. A similar tendency was also observed for mouse IgG antibodies ( Figure 3B ), and the relative IgG content is higher than the IgM content. In brief, despite the lower sugar density of the synthetic glycoconjugate, GH-DT has similar immunogenicity to GH-KLH, and α-GalCer adjuvant was shown to enhance the immune response.
[0191] Since C1 has been shown to b...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap