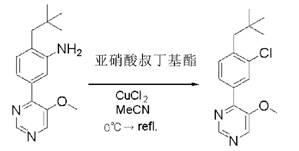
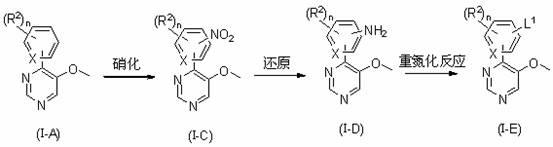
Pyrimidine derivatives, horticultural insecticides comprising said derivatives and method of use thereof
A technology of pyrimidine derivatives, C1-C6, applied in the directions of pesticides, botanical equipment and methods, medical preparations containing active ingredients, etc., can solve problems such as no teaching or disclosure of insecticidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1152] (1-1) Production of 2-chloro-4-(4-tert-butylphenyl)-5-methoxypyrimidine
[1153]
[1154] Combine 4-tert-butylphenylboronic acid (3.98 g, 0.022 mol), sodium carbonate (7.00 g, 0.066 mol), 2,4-dichloro-5-methoxypyrimidine (4.0 g, 0.022 mol) and tetrakis ( Triphenylphosphine) palladium (2.54 g, 0.0022 mol) was added to a mixed solvent of acetonitrile (60 mL) and water (40 mL). The reaction vessel was replaced with argon, and the mixture was reacted under reflux with heating for 3 hr. The mixture was cooled to room temperature and poured into water. The mixture was extracted with methyl tert-butyl ether and washed with saturated brine. The organic layer was dried with anhydrous magnesium sulfate, and the solvent was distilled off. The residue was purified by silica gel column chromatography to provide the target product (4.33 g),
[1155] Yield: 71%
[1156] Physical properties: melting point m.p. = 77-79°C.
[1157] (1-2) Preparation of 4-(4-tert-butylphenyl)-5-methoxypyrimi...
Embodiment 2
[1162] Example 2. Preparation of 4-(4-tert-butylphenyl)-5-hydroxypyrimidine (Compound No. 1-1)
[1163]
[1164] Add 4-(4-tert-butylphenyl)-5-methoxypyrimidine (1.40 g, 5.78 mmol) and lithium iodide (2.48 g, 18.5 mmol) to 2,4,6-trimethylpyridine ( 6 mL), react under heating and reflux for 8 hr. The mixture was cooled to room temperature and poured into 3N hydrochloric acid (30 ml). The mixture was extracted with ethyl acetate. The organic layer was dried with anhydrous magnesium sulfate, and the solvent was distilled off. The residue was purified by silica gel column chromatography to provide the target product (0.73 g),
[1165] Yield: 55%
[1166] Physical properties: melting point m.p. = 104-106°C.
Embodiment 3
[1167] Example 3. Preparation of 4-(4-tert-butylphenyl)-5-n-propoxypyrimidine (Compound No. 1-86)
[1168]
[1169] Add 4-(4-tert-butylphenyl)-5-hydroxypyrimidine (0.37 g, 1.62 mmol), n-propanol (0.18 g, 2.43 mmol) and triphenylphosphine (0.64 g, 2.43 mmol) to tetrahydrofuran (5 mL), and diethyl azodicarboxylate (1.06 g, 2.43 mmol) was added dropwise to the solution. Then, the mixture was reacted at room temperature for 2 hr. The reaction solution was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography to provide the target product (0.27 g),
[1170] Yield: 62%
[1171] Physical properties: refractive index n D = 1.5618 (25.5°C).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap