Vanadium electrolyte of battery and preparation method thereof
An electrolyte and vanadium battery technology, applied in the field of vanadium battery electrolyte and its preparation, can solve the problems of no charge and discharge test report, low test current density, valence mismatch and accumulation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1.8M vanadyl sulfate-sulfuric acid solution (VOSO 4 , purchased from the market) added to 3M's H 2 SO 4100 mL of a blank electrolyte solution with a vanadium ion concentration of 2.0 M was prepared. At room temperature, add 0.3 g of acesulfame K, the concentration of which is 1.0% by mass, and keep stirring until the acesulfame K is completely dissolved. The acesulfame potassium used in this embodiment is purchased from the market. Through the above steps, the electrolyte solution for vanadium battery provided by the invention is prepared.
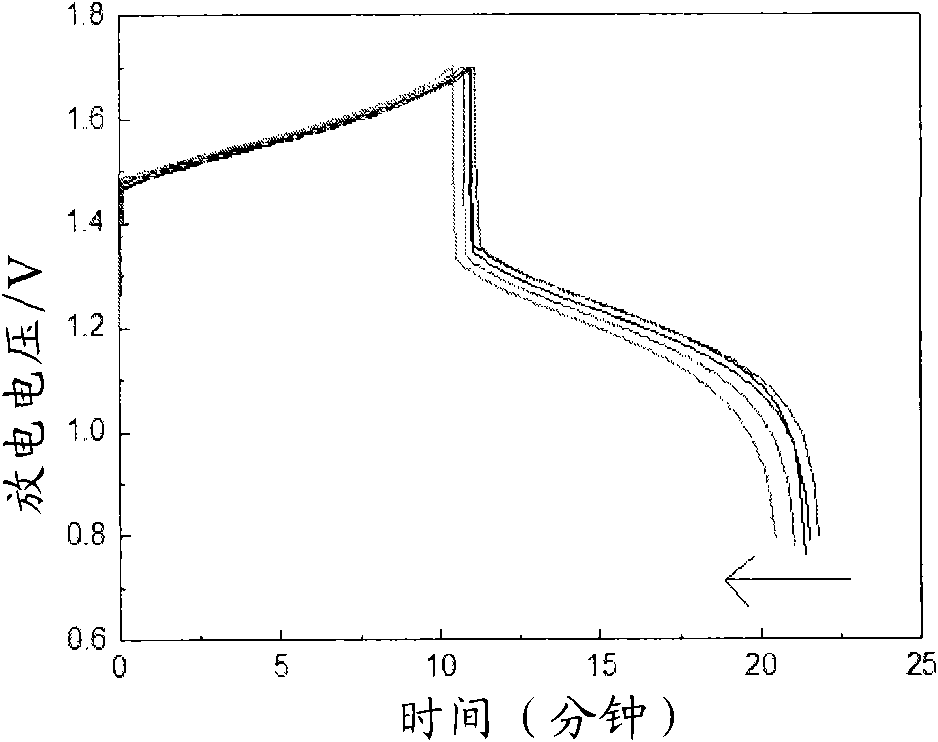
[0027] The above-mentioned electrolyte is placed in the positive and negative electrolyte storage tanks of the vanadium battery respectively according to the ratio of volume ratio 2: 1, with graphite felt as the electrode, and the cation exchange membrane (perfluorosulfonic acid membrane) as the battery diaphragm. The current density is 30-80mA / cm 2 The charge-discharge activation is carried out at a current density of 1.5 mol / ...
Embodiment 2
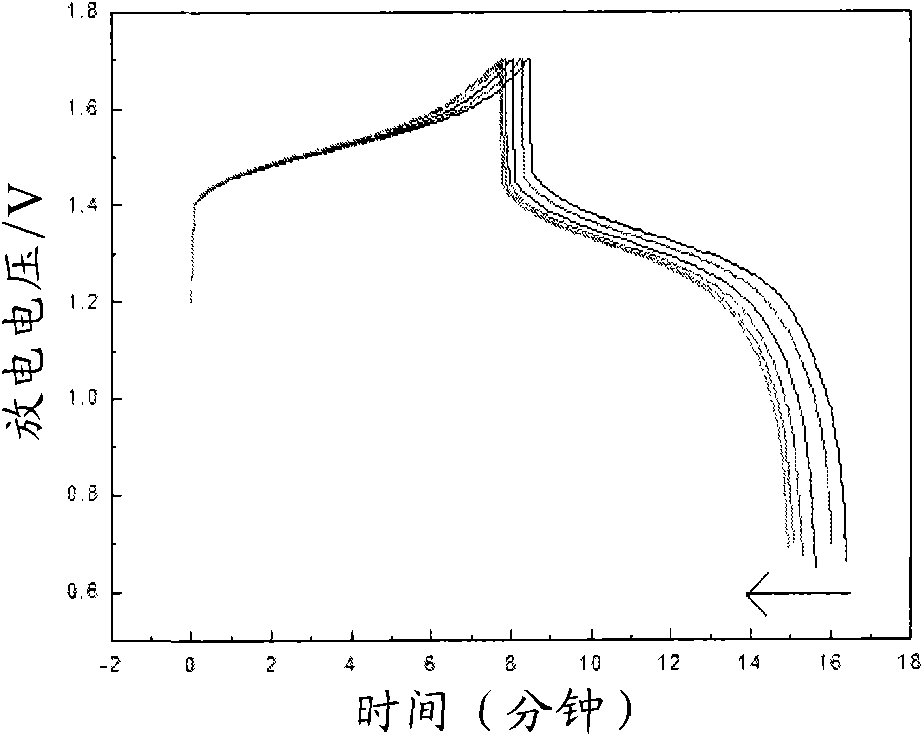
[0031] With 1.8M VOSO 4 solution with 3M H 2 SO 4 500mL of the solution is the electrolyte, placed in the cathode of the electrolytic cell, with 3mol / L sulfuric acid system as the anode, the electrode material is a lead plate, and the perfluorosulfonic acid ion exchange membrane is the diaphragm, at 60-250mA / cm 2 Charging is carried out under the current density, and at the same time, 3.5 g of acesulfame potassium is added to the cathode of the electrolytic cell for electrolysis, and its mass concentration is 2.4%. The end point of electrolysis is controlled by detecting the potential of the electrolyte, the end point potential is 150-250Mv, and the vanadium ion concentration of 1.6mol / L is obtained for the positive and negative electrodes of the all-vanadium redox flow battery. 3+ / V 4+ electrolyte. The obtained electrolyte is used in a vanadium battery, at a certain 50mA / cm 2 The battery was charged and discharged under the current density. The initial discharge voltage...
Embodiment 3-7
[0033] Table 1 shows the addition method adopted in Examples 3-7, the type and concentration of the addition, and the battery performance obtained from the test.
[0034] Table 1
[0035]
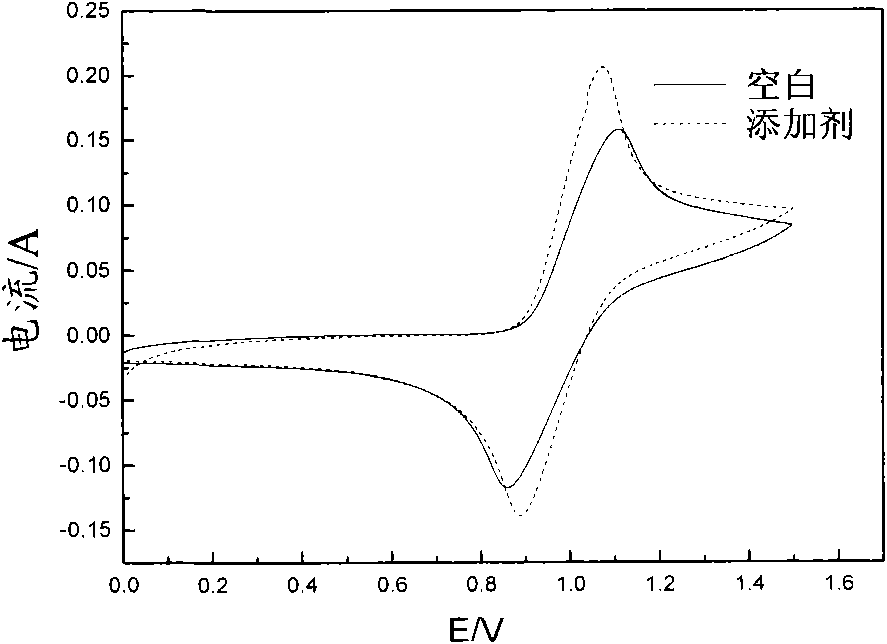
[0036] It can be seen from the above data and drawings that the present invention has the advantage of selecting a class of water-soluble organic additives to improve the activity of the electrolyte and the reversibility of redox reactions. Moreover, the selected additives are low in dosage, easy to obtain, and low in cost; the additive itself has good stability, high water solubility, and good stability to heat and acid, thereby improving the cycle stability of the electrolyte, suppressing battery capacity decay, and reducing Electrolyte sensitivity to temperature.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com