Composition comprising the purified extract of bee venom for preventing and treating degenerative brain diseases
A technology of degenerative encephalopathy and extracts, applied in the direction of drug combination, medical raw materials derived from arthropods, drug delivery, etc., can solve the therapeutic effect or improvement effect of unpurified bee venom extracts on brain degenerative diseases, report or publish, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Preparation of crudely purified extract of bee venom
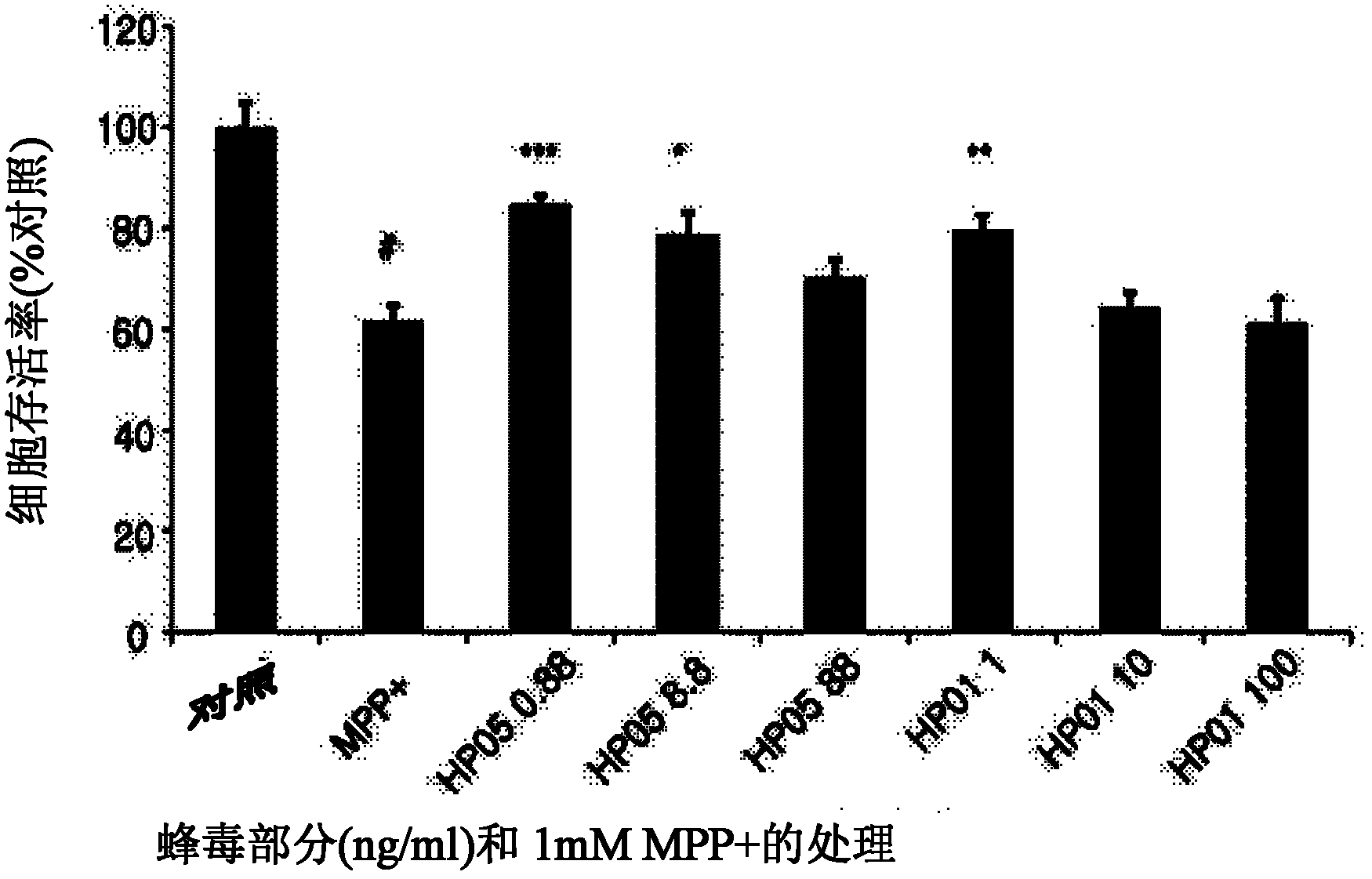
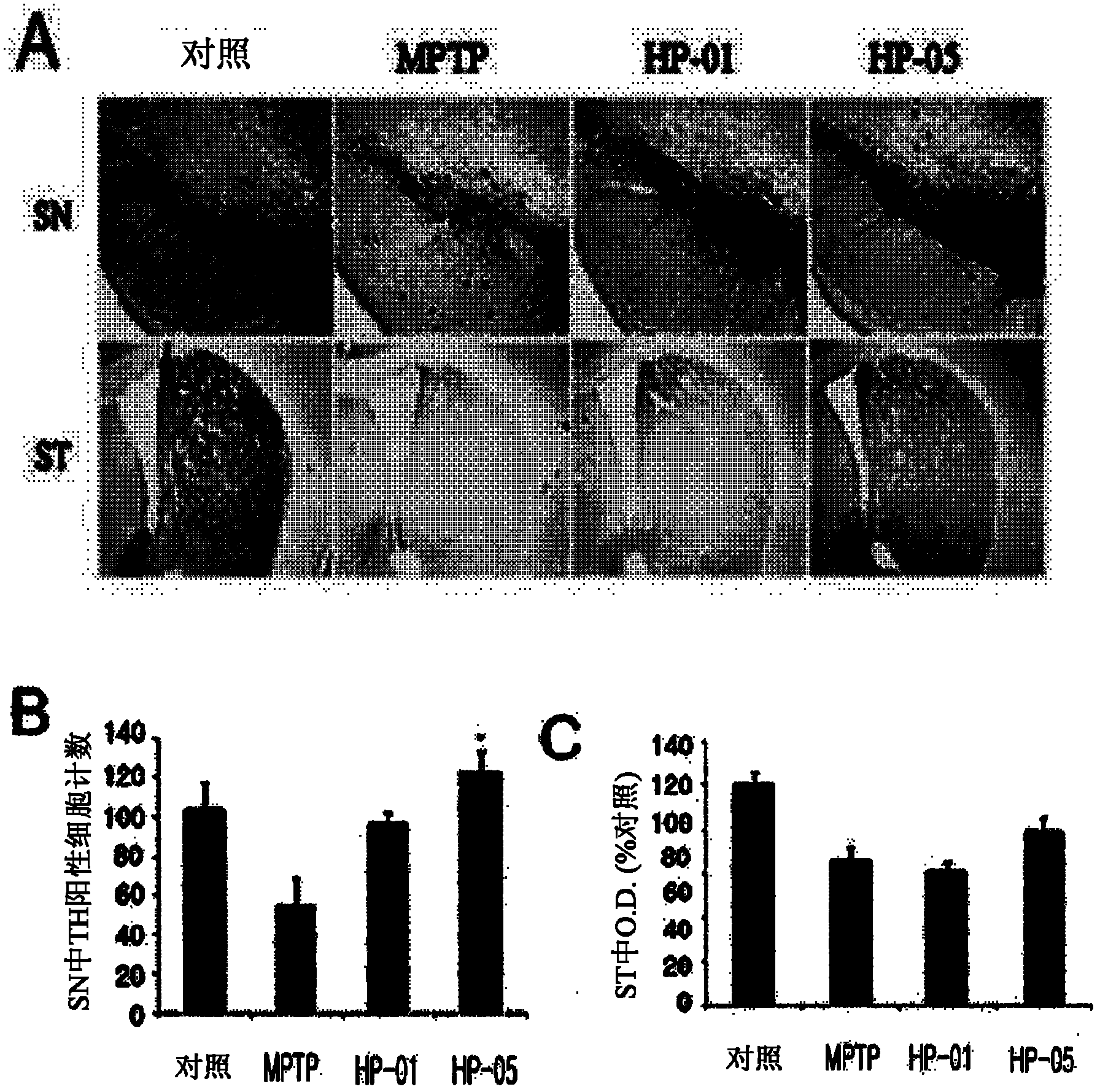
[0066] 10.0 g of dried bee venom collected from honeybees was dissolved in water, and impurities were removed by filtration using a syringe filter (Minisart RC 15, 0.20 micron, Sartorius Co. Germany). The filtrate was dried with a lyophilizer (FDCF-12012, Operon Co. Korea) to obtain 9.76 g of dried crude purified extract of meli venom (hereinafter designated as "HP-01"). The dry powder was used as a test sample in the following experiments.
Embodiment 2
[0067] Example 2: Preparation of Purified Extract of Bee Venom Using Gel Filtration Chromatography
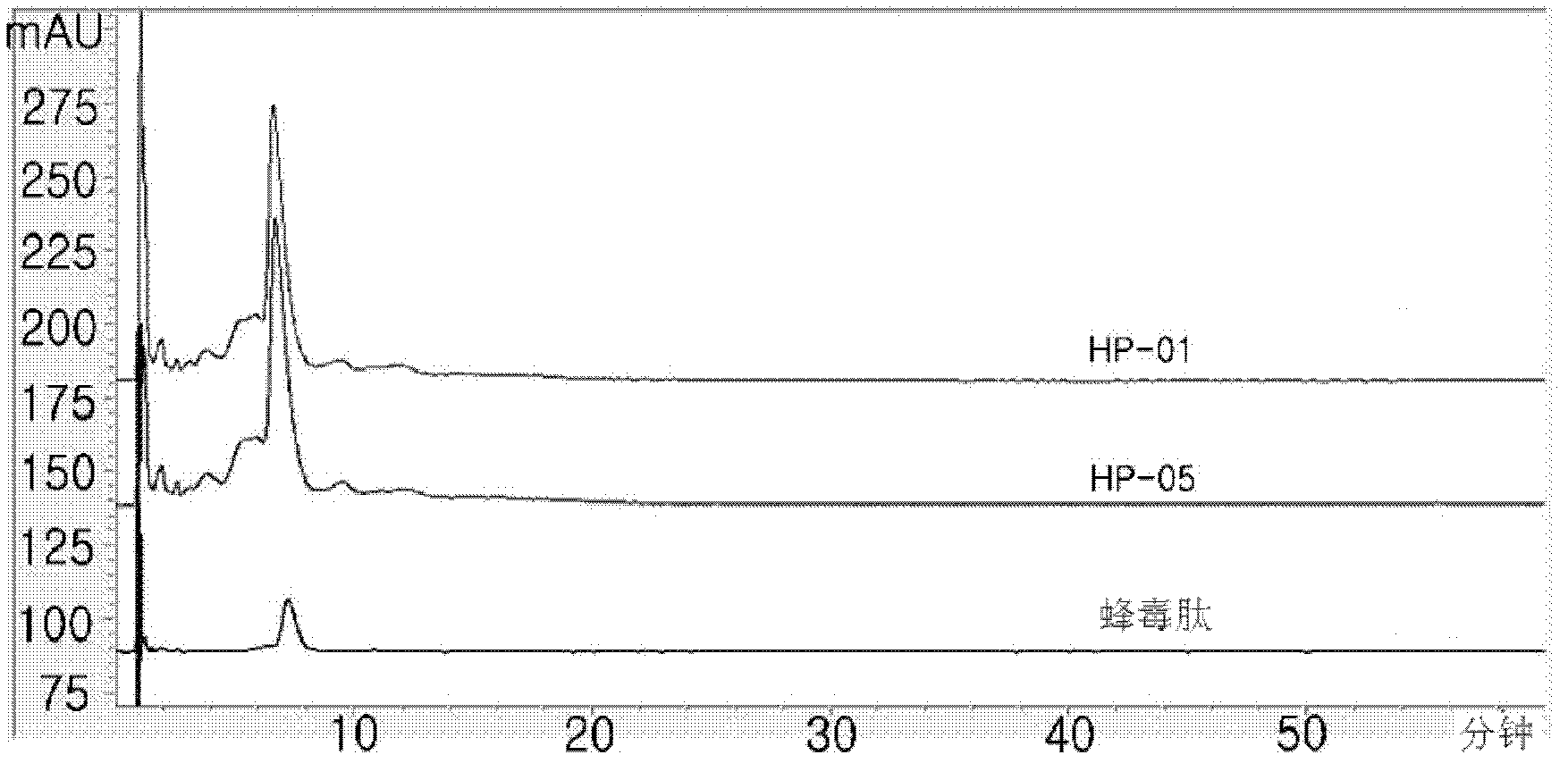
[0068] 100 mg of the dry crude purified extract prepared in Example 1 was dissolved in 1.0 ml of distilled water (HPLC grade) and subjected to gel filtration chromatography according to the conditions disclosed in Table 1 to obtain 20 fractions. Each fraction was added to a protein dialysis membrane (Spectra / por 7, Spectrum Co. USA) and the membrane was immersed in a cylindrical glass flask containing 500 ml of distilled water (HPLC grade) for dialysis with stirring for 90 minutes. After completion of the dialysis desalination process, the desalted solution present in the membrane was lyophilized using a lyophilizer (FDCF-12012, Operon Co. Korea) for 3 days and each of the purified fractions was collected to obtain 74 mg of bee venom purified extract (yield : 74%) (hereinafter designated as "HP-01G").
[0069] Table 1
[0070]
[0071] The compositions of HP-01 and HP-01G ...
Embodiment 3
[0075] Example 3: Preparation of a purified extract of bee venom using the salting-out method
[0076] 100 mg of the dry crude purified extract prepared in Example 1 was dissolved in 5.0 ml of distilled water (HPLC grade) to adjust to 20 mg / ml, and the solution was subjected to a salting process at room temperature by adding ammonium sulfate dropwise with stirring for 1 hour to form a 30% ammonium sulfate solution. The solution was further stirred at room temperature for 1 hour and subjected to a salting-out process by dropwise addition of ammonium sulfate to form an 80% solution.
[0077] The solution was left alone at 0° C. for 2 hours to provide sufficient time for the sufficient salting-out process, and centrifuged at 15000 rpm for 15 minutes by using an ultracentrifuge (Ultra 5.0, Hanil Science Medical Co. Ltd, Korea). The supernatant was collected and the pellet was dissolved in 5 ml of distilled water (HPLC grade) to desalt and lyophilize each to give 19 mg of a purifi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com