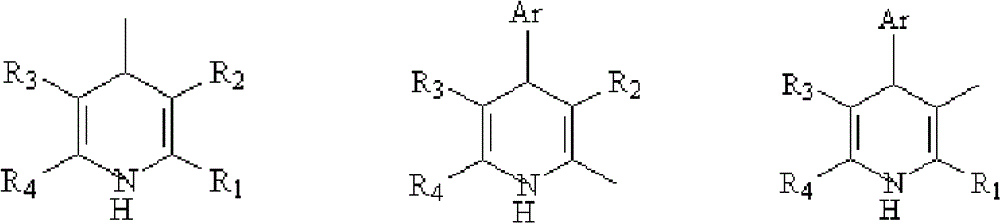
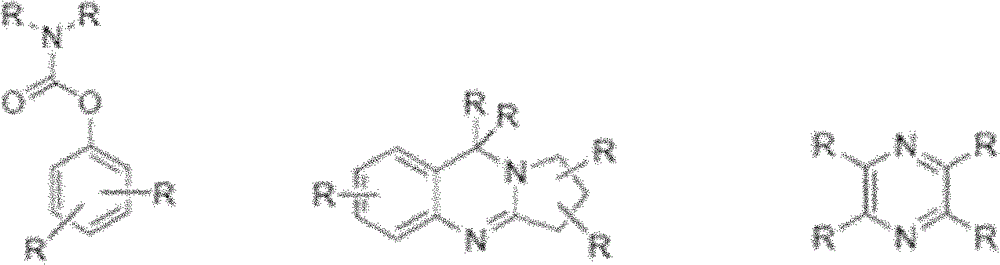
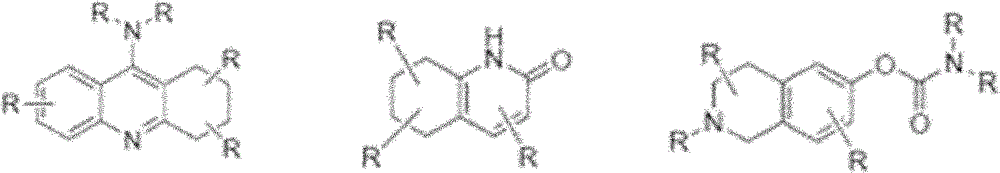
Compounds and their use as l-type calcium channel blockers or/and acetylcholinesterase inhibitors
A compound, ethyl technology, applied in the field of chemical synthesis, can solve problems such as the lack of acetylcholine, the failure of nerve signal transmission, affecting the body's cognition and memory, and achieve obvious inhibitory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] Embodiment 1 prepares compound 1 provided by the invention
[0144] The preparation process is as follows:
[0145]
[0146] Accurately weigh 3.0g of about 24.6mmol of 3-hydroxybenzaldehyde, dissolve it in 100mL of acetone, stir to dissolve, add 6.8g, equivalent to 50mmol of K 2 CO 3 Solid powder, stirred vigorously, and then slowly added dropwise 2.75g (25.6mmol) of N,N-dimethylformyl chloride, a small amount of bubbles were generated. After the reaction is stable, move the reactor into a 60°C oil bath and heat to reflux, TLC detection, stop the reaction after 21h, cool, filter, discard the filter residue, evaporate the filtrate to dryness and add saturated NaHCO 3 The solution was diluted, left to stand and separated and extracted with ethyl acetate, the organic extract was collected, washed with water, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain an oily substance, which was purified by silica gel column chroma...
Embodiment 2
[0151] Embodiment 2 prepares compound 2 provided by the invention
[0152] The preparation process is as follows:
[0153]
[0154] Accurately weigh 2.0 g of isopropyl acetoacetate, dissolve it in 15 mL of absolute ethanol, then add 1.6 g of about 20.83 mmol of ammonium acetate, heat to reflux in an oil bath at 90°C, detect by TLC, stop the reaction after 24 hours, evaporate under reduced pressure at low temperature dry reaction solution, to obtain compound S 1 2.0 g, yield 100%. ESI-MS[M+H] + = 144.1.
[0155] Weigh 1.0g of about 4.0mmol of 3-hydroxybenzaldehyde, dissolve it in 50mL of acetone, stir to dissolve, then add 2.3g of about 16.3mmol of K 2 CO 3 Solid powder, after stirring vigorously, slowly add 0.5g of about 4.5mmol of N,N-dimethylformyl chloride dropwise, a small amount of bubbles are generated, after the reaction is stable, heat and reflux in an oil bath at 60°C, detect by TLC, stop after 12h React, cool, filter, discard the filter residue, collect the ...
Embodiment 3
[0161] Embodiment 3 prepares compound 3 provided by the invention
[0162] The preparation process is as follows:
[0163]
[0164] Accurately weigh 0.5g of 3-nitro-4-hydroxybenzaldehyde equivalent to 2.99mmol, dissolve it in 5mL of anhydrous pyridine solution, stir to dissolve, then slowly add 0.38g of about 3.55mol of N,N-dimethyl Phyloyl chloride, a small amount of bubbles are generated, after the reaction is stable, let it stand at room temperature, TLC detection, stop the reaction after 14h, there is solid precipitation, filter and discard the filter residue, collect the filtrate and dilute with ethyl acetate, then add dropwise 1mol / L of hydrochloric acid to adjust the pH value to >7, extract the organic layer, wash with water, dry over anhydrous sodium sulfate, filter, and concentrate under reduced pressure to obtain a white solid, which is purified by silica gel column chromatography, using the volume of petroleum ether and acetone The eluent with a ratio of 2:1 was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com