Albumin-modified PAMAM self-assembly transgenic composition, preparation method and application thereof
A composition and transgenic technology, applied in gene therapy, pharmaceutical formulations, genetic material components, etc., can solve the problems of increasing the steric hindrance of the combination of PAMAM molecules and DNA molecules, reducing transfection efficiency, and improving cytotoxicity, etc. Effects of toxicity and hemolysis, reduction of positive charge intensity, improvement of biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
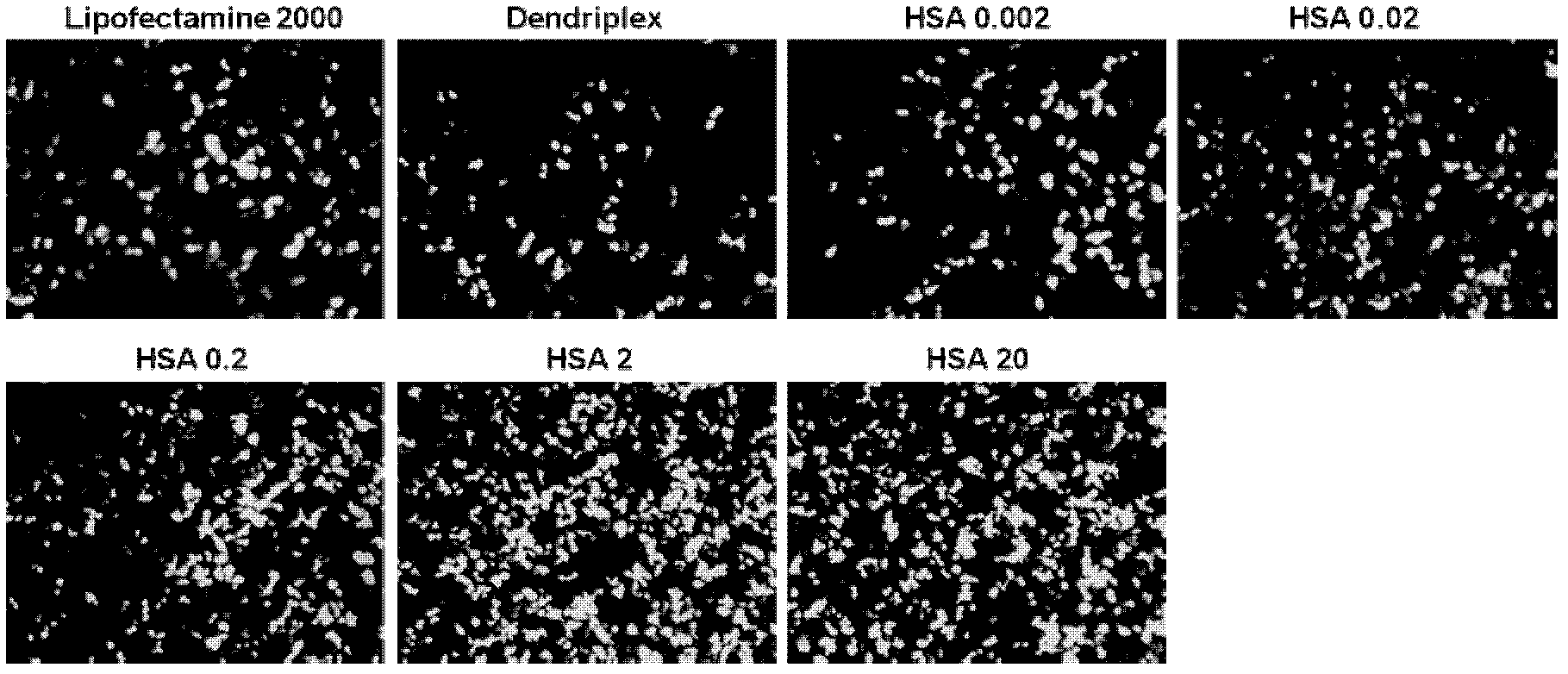
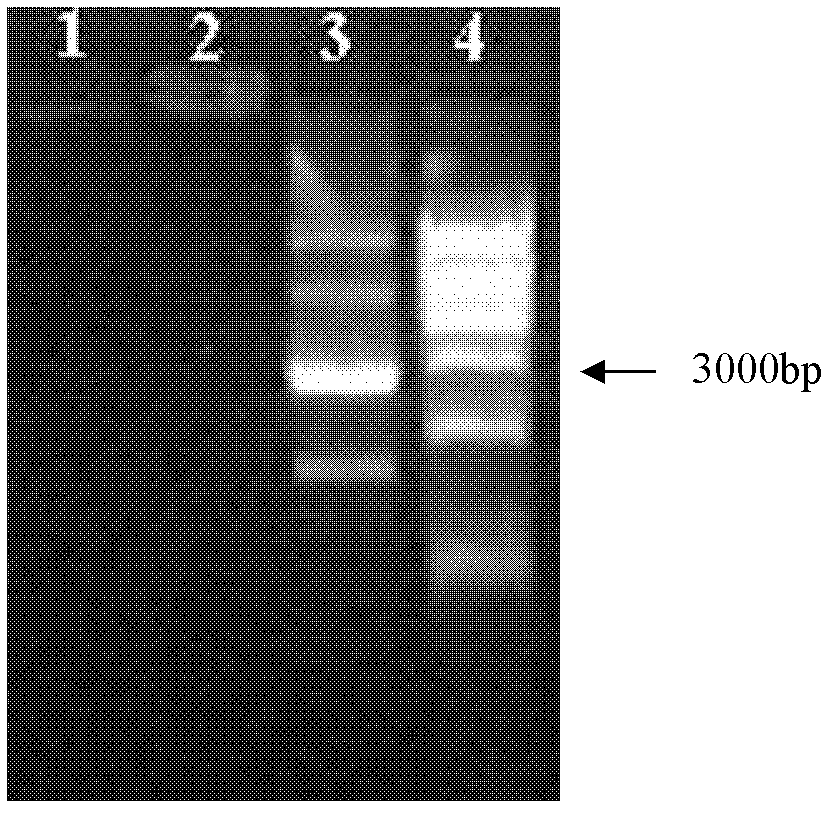
[0037] Embodiment 1, preparation HSA / PAMAM / DNA complex and its characterization
[0038] 1. Screening of different PAMAM algebras and different charge ratios
[0039] 1) Dissolve the 10 mg / mL storage solutions of PAMAM G3, PAMAM G4 and PAMAM G5 (number average molecular weights: 6909, 14215 and 28826, respectively) prepared above in sterile distilled water, shake and mix well, and prepare 1 mg / mL Working solution, stored at 4°C for future use. Before transfection, take 1.0 μg pGL-3.0 plasmid DNA (pGL-3.0 plasmid was purchased from Promega Company, catalog number: E1771) and put it into EP tubes, add 0.7 μL, 1.4 μL, 3.5 μL, and 7 μL respectively at a concentration of 1 mg / Add 500uL of opti-MEM medium (purchased from invitrogen, catalog number 11058-021) to 5 mL of PAMAM G3, PAMAM G4 and PAMAM G5 working solutions, mix well and incubate at room temperature for 10 min to form a PAMAM / DNA complex. In the PAMAM / DNA complex, the positive and negative charge ratios of PAMAM and DNA ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap