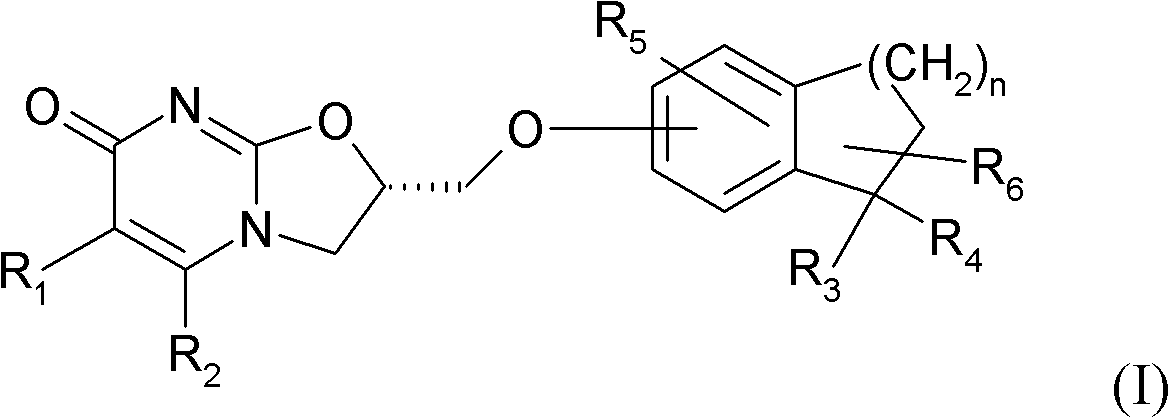
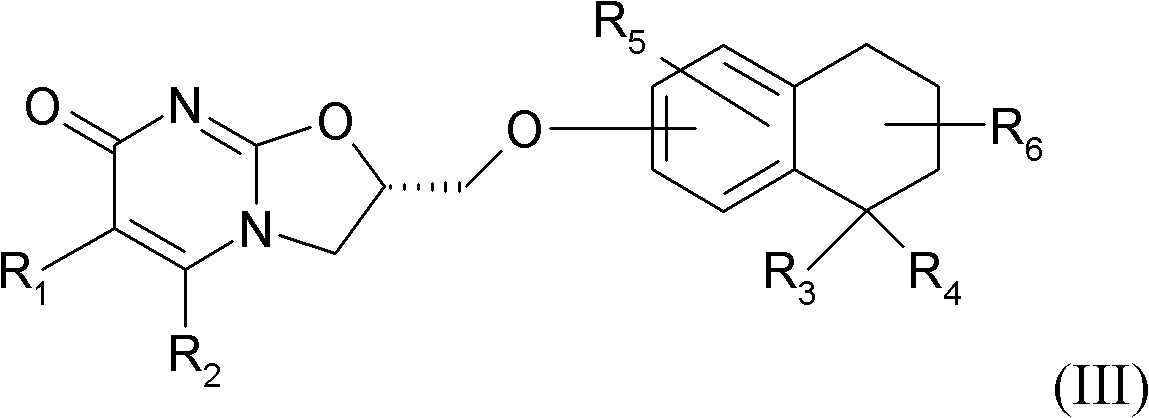
Substituted dihydro benzocycloalkyloxymethyl oxazolopyrimidinones, preparation and use thereof
一种二氢噁唑、基氧基甲基的技术,应用在制备这些化合物领域,能够解决无活性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0175] (S)-2-(1,1-Dimethylindan-5-yloxymethyl)-2,3-dihydrooxazolo[3,2-a]pyrimidin-7-one
[0176]
[0177] Step 1: 1,1-Dimethylindan-5-ol
[0178]The title compound was prepared in two steps following the procedure described in US Patent Application Publication No. 2006100460, using 5-methoxyindan-1-one as a starting material.
[0179] C 11 h 14 O(162.10), LCMS(ESI): 163.11(M + +H).
[0180] 1 HNMR (CDCl 3 , 300MHz), δ6.98(d, 1H), 6.66(s, 1H), 6.64(d, 1H), 4.51(s, 1H), 2.83(t, 2H), 1.92(t, 2H), 1.23( s, 6H).
[0181] Step 2: (S)-2-(1,1-Dimethylindan-5-yloxymethyl)-2,3-dihydrooxazolo[3,2-a]pyrimidin-7-one
[0182] In (S)-2-toluene-4-sulfonic acidmethyl-2,3-dihydrooxazolo[3,2-a]pyrimidin-7-one ((S)-2-toluene-4-sulfonic acidmethyl -2,3-dihydro-oxazolo[3,2-a]pyrimidin-7-one) (0.8g, 2.48mmol) (prepared according to the operation described in WO 2008 / 112483) in acetonitrile (80ml) was added 1 , 1-Dimethylindan-5-ol (0.4 g, 2.48 mmol), then cesium carbonate (0.82 g, 2.48 ...
Embodiment 2
[0186] (S)-2-(5,5-dimethyl-5,6,7,8-tetrahydronaphthalen-2-yloxymethyl)-2,3-dihydrooxazolo[3,2- a] pyrimidin-7-one
[0187]
[0188] Step 1: 5,5-Dimethyl-5,6,7,8-tetralin-2-ol
[0189] The title compound was prepared in two steps following the procedure described in US Patent Application Publication No. 2006100460, using 6-methoxy-3,4-dihydro-2H-naphthalen-1-one as starting material.
[0190] C 12 h 16 O(176.12), LCMS(ES + ): 177.14 (M + +H).
[0191] 1 H NMR (CDCl 3 , 300MHz), δ7.19(d, 1H), 6.64(dd, 1H), 6.51(d, 1H), 4.46(s, 1H), 2.70(t, 2H), 1.79(m, 2H), 1.63( m, 2H), 1.26 (s, 6H).
[0192] Step 2: (S)-2-(5,5-Dimethyl-5,6,7,8-tetrahydronaphthalen-2-yloxymethyl)-2,3-dihydrooxazolo[3 , 2-a] pyrimidin-7-one
[0193] Using the procedure described in Example 1, the title compound was prepared from 5,5-dimethyl-5,6,7,8-tetralin-2-ol. [α] D 25 =-58.0 (c=0.87, CHCl 3 ).
[0194] C 19 h 22 N 2 o 3 (326.16), LCMS (ES + ): 327.16 (M + +H).
[0195] 1 H NMR (CD...
Embodiment 3
[0197] (S)-2-(5,5-Dimethyl-6,7,8,9-tetrahydro-5H-benzocyclohepten-2-yloxymethyl)-2,3-dihydrooxa Azolo[3,2-a]pyrimidin-7-one
[0198]
[0199] Step 1: 5,5-Dimethyl-6,7,8,9-tetrahydro-5H-benzocyclohepten-2-ol
[0200] The title compound was prepared according to the procedure described in U.S. Patent Application Publication No. 2006100460, using 2-methoxy-6,7,8,9-tetrahydro-benzocyclohepten-5-one as a starting material, and two prepared in steps.
[0201] C 13 h 18 O(190.14), LCMS(ES + ): 191.15 (M + +H).
[0202] 1 H NMR (CDCl 3 , 300MHz), δ7.22(d, 1H), 6.59(dd, 1H), 6.57(s, 1H), 4.49(s, 1H), 2.87(m, 2H), 1.84(m, 2H), 1.64( m, 4H), 1.35 (s, 6H).
[0203] Step 2: (S)-2-(5,5-Dimethyl-6,7,8,9-tetrahydro-5H-benzocyclohepten-2-yloxymethyl)-2,3- Dihydrooxazolo[3,2-a]pyrimidin-7-one
[0204] Using the procedure described in Example 1, the title compound was prepared from 5,5-dimethyl-6,7,8,9-tetrahydro-5H-benzocyclohepten-2-ol. [α] D 25 =-64.4 (c=0.68, CHCl 3 ).
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com