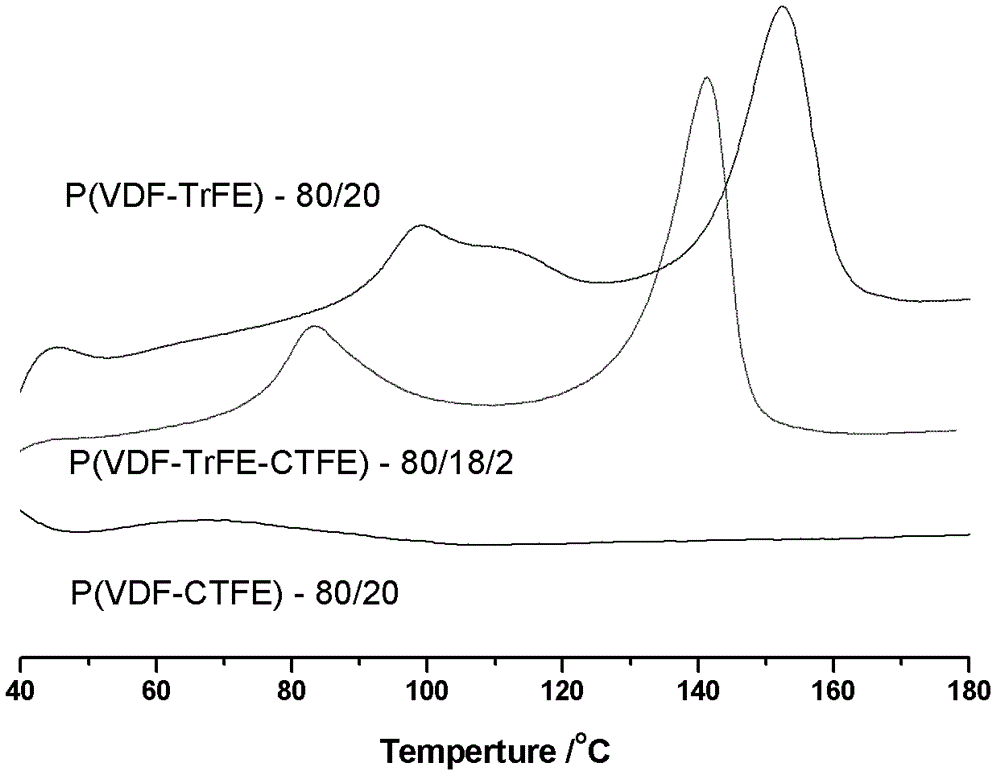
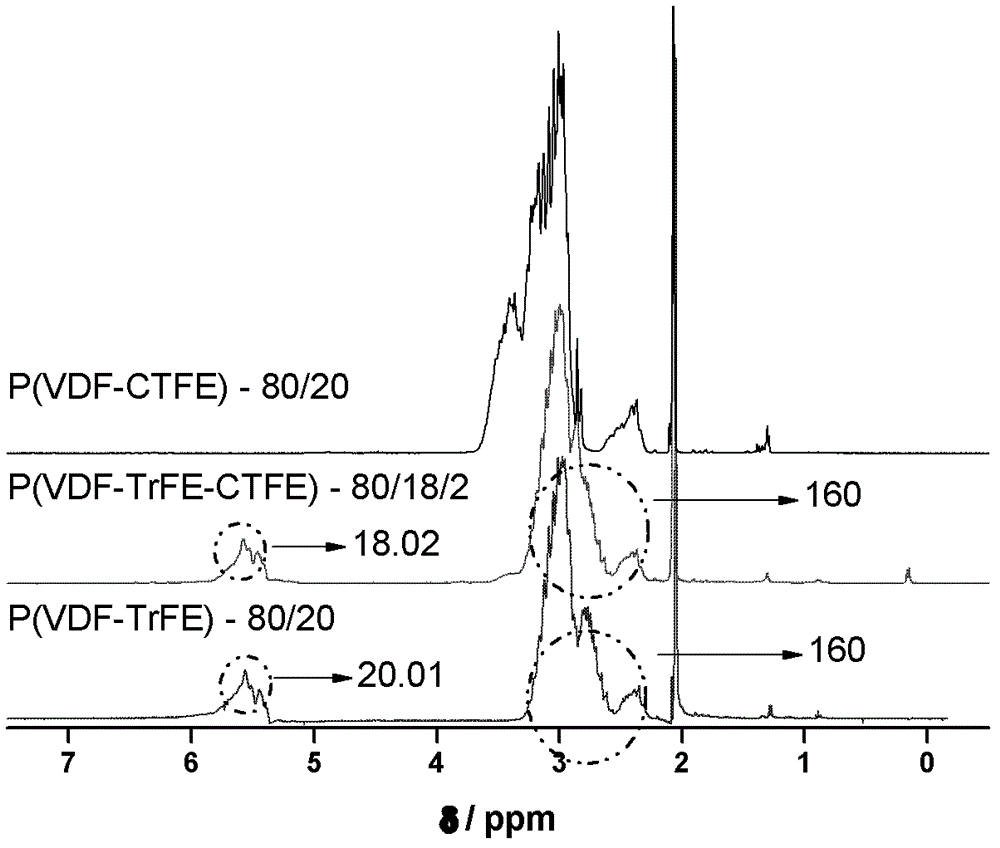
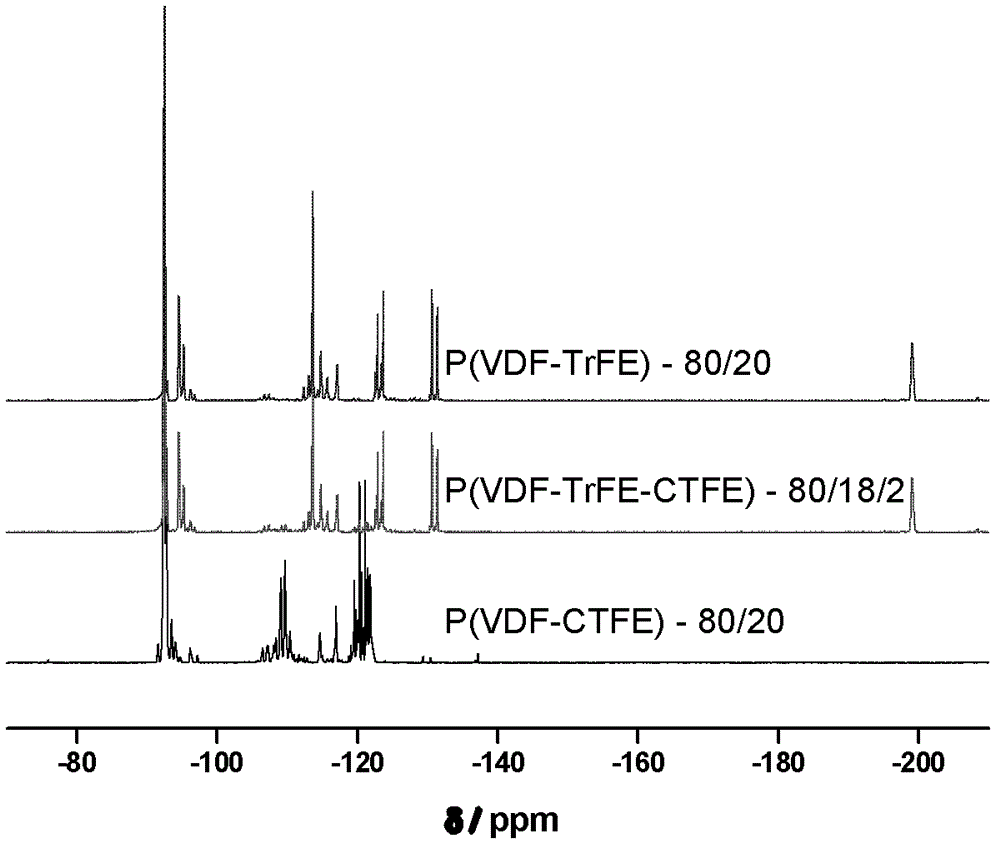
Method for preparing poly(vinylidene fluoride-trichloroethylene) or poly(vinylidene fluoride-chlorotrifluoroethylene-trichloroethylene)
A technology of chlorotrifluoroethylene and vinylidene fluoride, which is applied in the field of preparing poly(vinylidene fluoride-trifluoroethylene) or poly(vinylidene fluoride-trifluorochloroethylene-trifluoroethylene), can solve the problem of fluoropolymer elimination Degradation, difficult to remove copper salts and other problems, to achieve the effect of low cost, friendly to the human body and the environment, and the method is simple and easy to control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] A method for preparing poly(vinylidene fluoride-chlorotrifluoroethylene-trifluoroethylene) by reduction of poly(vinylidene fluoride-chlorotrifluoroethylene), comprising the following steps:
[0020] Step 1. In the three-necked bottle, add poly(vinylidene fluoride-chlorotrifluoroethylene) P(VDF-CTFE), the molar composition is VDF / CTFE=80:20, and simultaneously add a solvent, poly(vinylidene fluoride-trifluoroethylene) The mass ratio of poly(vinylidene fluoride-chlorotrifluoroethylene) P(VDF-CTFE) to solvent is 4.5:100. After the poly(vinylidene fluoride-chlorotrifluoroethylene) P(VDF-CTFE) is fully dissolved, a reducing agent is added. The molar ratio of Cl atoms in (vinylidene fluoride-chlorotrifluoroethylene) P(VDF-CTFE) is 3.4:1, and after the reducing agent is dissolved by magnetic stirring, the stirring reaction is continued at room temperature for 20 hours;
[0021] Described solvent is tetrahydrofuran;
[0022] The reducing agent is LiAlH 4 ;
[0023] Step 2, p...
Embodiment 2
[0027] A method for preparing poly(vinylidene fluoride-chlorotrifluoroethylene-trifluoroethylene) by reduction of poly(vinylidene fluoride-chlorotrifluoroethylene), comprising the following steps:
[0028]Step 1. In the three-necked bottle, add poly(vinylidene fluoride-chlorotrifluoroethylene) P(VDF-CTFE), the molar composition is VDF / CTFE=80:20, and simultaneously add a solvent, poly(vinylidene fluoride-trifluoroethylene) The mass ratio of poly(vinylidene fluoride-chlorotrifluoroethylene) P(VDF-CTFE) to the solvent is 3.6:100. After the poly(vinylidene fluoride-chlorotrifluoroethylene) P(VDF-CTFE) is fully dissolved, a reducing agent is added. The molar ratio of Cl atoms in (vinylidene fluoride-chlorotrifluoroethylene) P(VDF-CTFE) is 2.7:1, and after the reducing agent is dissolved by magnetic stirring, the stirring reaction is continued at room temperature for 20 hours;
[0029] The solvent is dimethyl sulfoxide;
[0030] The reducing agent is LiAlH 4 ;
[0031] Step 2, p...
Embodiment 3
[0034] A method for preparing poly(vinylidene fluoride-chlorotrifluoroethylene-trifluoroethylene) by reduction of poly(vinylidene fluoride-chlorotrifluoroethylene), comprising the following steps:
[0035] Step 1. In the three-necked bottle, add poly(vinylidene fluoride-chlorotrifluoroethylene) P(VDF-CTFE), the molar composition is VDF / CTFE=80:20, and simultaneously add a solvent, poly(vinylidene fluoride-trifluoroethylene) The mass ratio of poly(vinylidene fluoride-chlorotrifluoroethylene) P(VDF-CTFE) to solvent is 4.5:100. After the poly(vinylidene fluoride-chlorotrifluoroethylene) P(VDF-CTFE) is fully dissolved, a reducing agent is added. The molar ratio of Cl atoms in (vinylidene fluoride-chlorotrifluoroethylene) P(VDF-CTFE) is 2.2:1, and after the reducing agent is dissolved by magnetic stirring, the stirring reaction is continued at room temperature for 20 hours;
[0036] Described solvent is tetrahydrofuran;
[0037] The reducing agent is NaBH 4 ;
[0038] Step 2, po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com