A mg2+, al3+, zr4+, f- ion co-doped garnet-type solid electrolyte
A solid electrolyte, garnet-type technology, used in circuits, electrical components, secondary batteries, etc., can solve the problem of garnet-type solid electrolyte lithium ion migration mechanism researchers clarifying and other problems, to increase the lithium ion migration channel cross section , strong electronegativity, the effect of improving conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
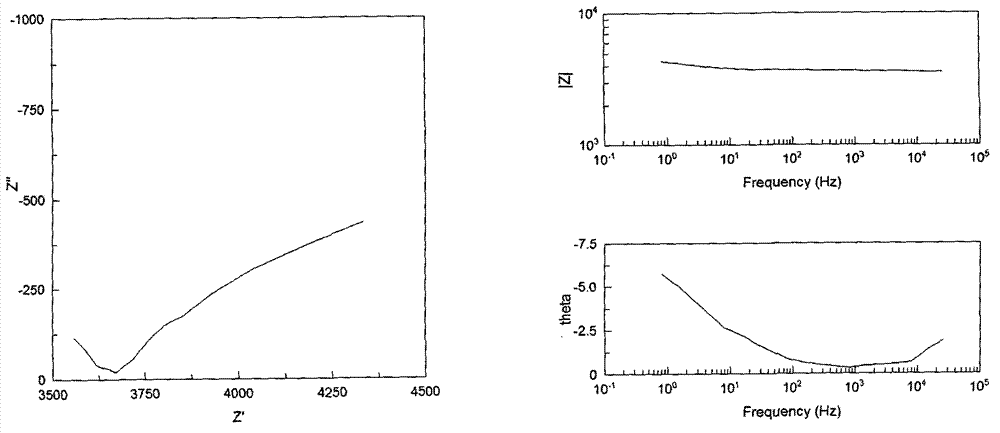
Image
Examples
Embodiment 1
[0011] Embodiment 1: Li 2 CO 3 : La 2 o 3 :MgO:Al 2 o 3 : ZrO 2 : Nb 2 o 5 : Li 2 F is 2.55: 1.41: 0.18: 0.06: 0.13: 0.875: 0.15 (molar ratio) ratio is evenly mixed, adds 3.2% 95% ethanol, ball mills 10 hours with the rotating speed of 250 rev / mins in ball mill, after ball mill finishes, Dry in a vacuum oven (vacuum degree 20 Pa) at 60°C for 10 hours, take it out and re-grind in an agate mortar for 30 minutes, heat up the ground powder to 720°C at a rate of 5°C / min and keep it for 10 hours, then heat it at 3°C Raise the temperature at a rate of 1 / min to 900°C for 11 hours to make a solid electrolyte powder. The powder is mixed with 2wt% binder PVC and kept under a pressure of 300MPa under a press for 5 minutes to form a thin sheet. The thin sheet is heated to 1000°C at a rate of 11°C / min and kept for 10 hours in an air atmosphere to make a lithium ion solid Electrolyte sheets.
Embodiment 2
[0012] Embodiment 2: Li 2 CO 3 : La 2 o 3 :MgO:Al 2 o 3 : ZrO 2 : Nb 2 o 5 : Li 2 F is that the ratio of 2.66: 1.3: 0.4: 0.07: 0.12: 0.87: 0.16 (molar ratio) mixes evenly, adds 8.5% 95% ethanol, ball mills 15 hours with the rotating speed of 380 rev / mins in ball mill, after ball mill finishes, Dry in a vacuum oven (vacuum degree 95Pa) at 80°C for 30 hours, take it out and re-grind in an agate mortar for 30 minutes, heat up the ground powder to 780°C at a rate of 8°C / min and keep it for 10 hours, then heat it at 7°C Raise the temperature at a rate of 1 / min to 950°C and keep it warm for 15 hours to make a solid electrolyte powder. The powder is mixed with 5wt% binder PVC and kept under a pressure of 450MPa under a press for 2 minutes to form a thin sheet. The thin sheet is heated to 1050°C at a rate of 15°C / min and kept for 10 hours in an air atmosphere to make a lithium-ion solid electrolyte. Flakes.
Embodiment 3
[0013] Embodiment 3: with Li 2 CO 3 : La 2 o 3 :MgO:Al 2 o 3 : ZrO 2 : Nb 2 o 5 : Li 2 F is 2.55: 1.45: 0.1: 0.05: 0.1: 0.9: 0.10 (molar ratio) ratio is evenly mixed, adds 3% 95% ethanol, ball mills 20 hours with the rotating speed of 300 rev / mins in ball mill, after ball mill finishes, Dry in a vacuum oven (vacuum degree 50Pa) at 70°C for 20 hours, take it out and re-grind in an agate mortar for 10 minutes, heat up the ground powder to 800°C at a rate of 10°C / min and keep it for 7 hours, then heat it at 2°C Raise the temperature at a rate of 1100°C for 12 hours to make solid electrolyte powder. The powder is mixed with 1wt% binder PVA and kept under a pressure of 300MPa under a press for 6 minutes to form a thin sheet. The thin sheet is heated to 1150°C at a rate of 15°C / min and kept for 28 hours in an air atmosphere to make a lithium ion solid Electrolyte sheets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com