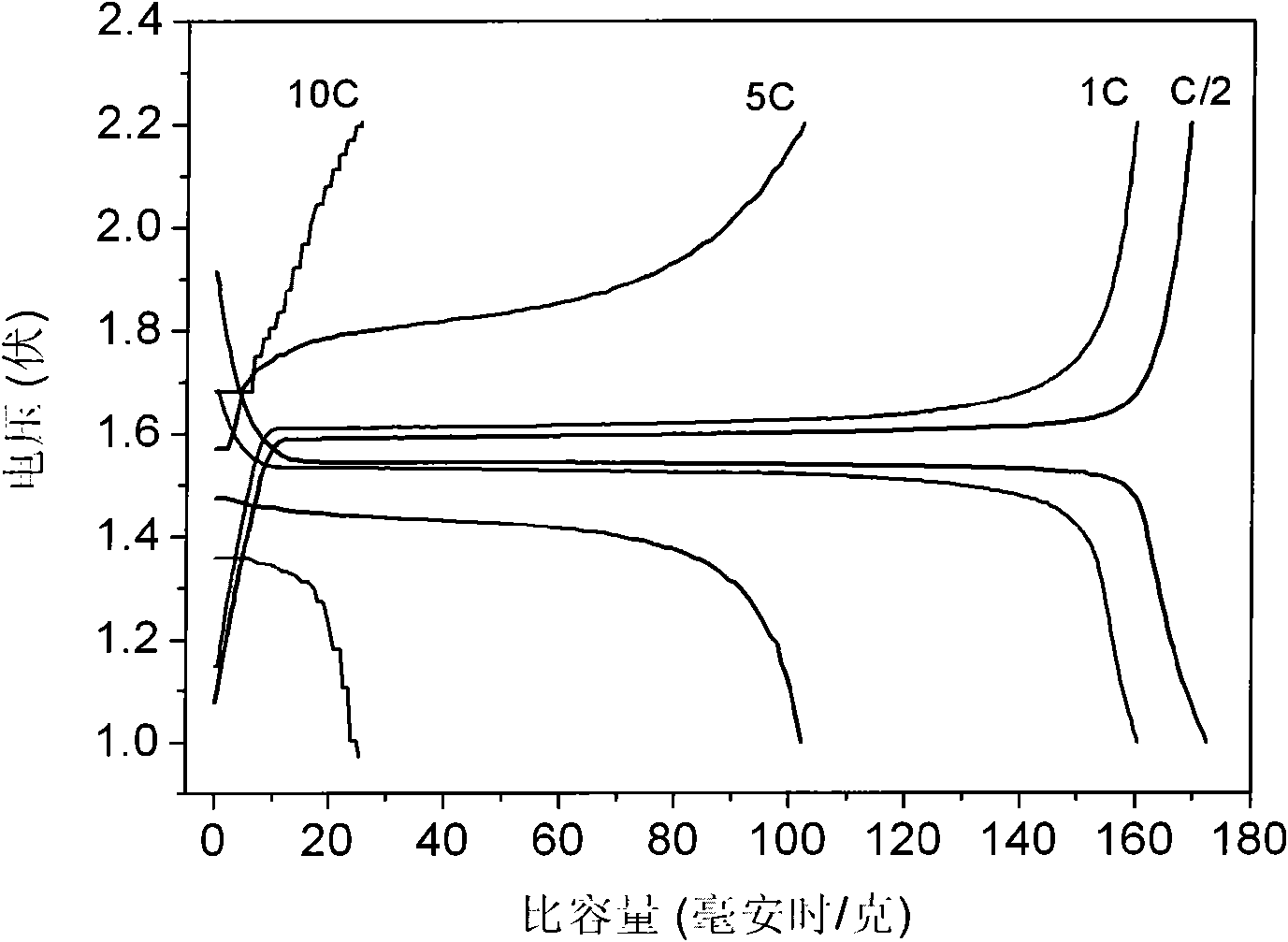
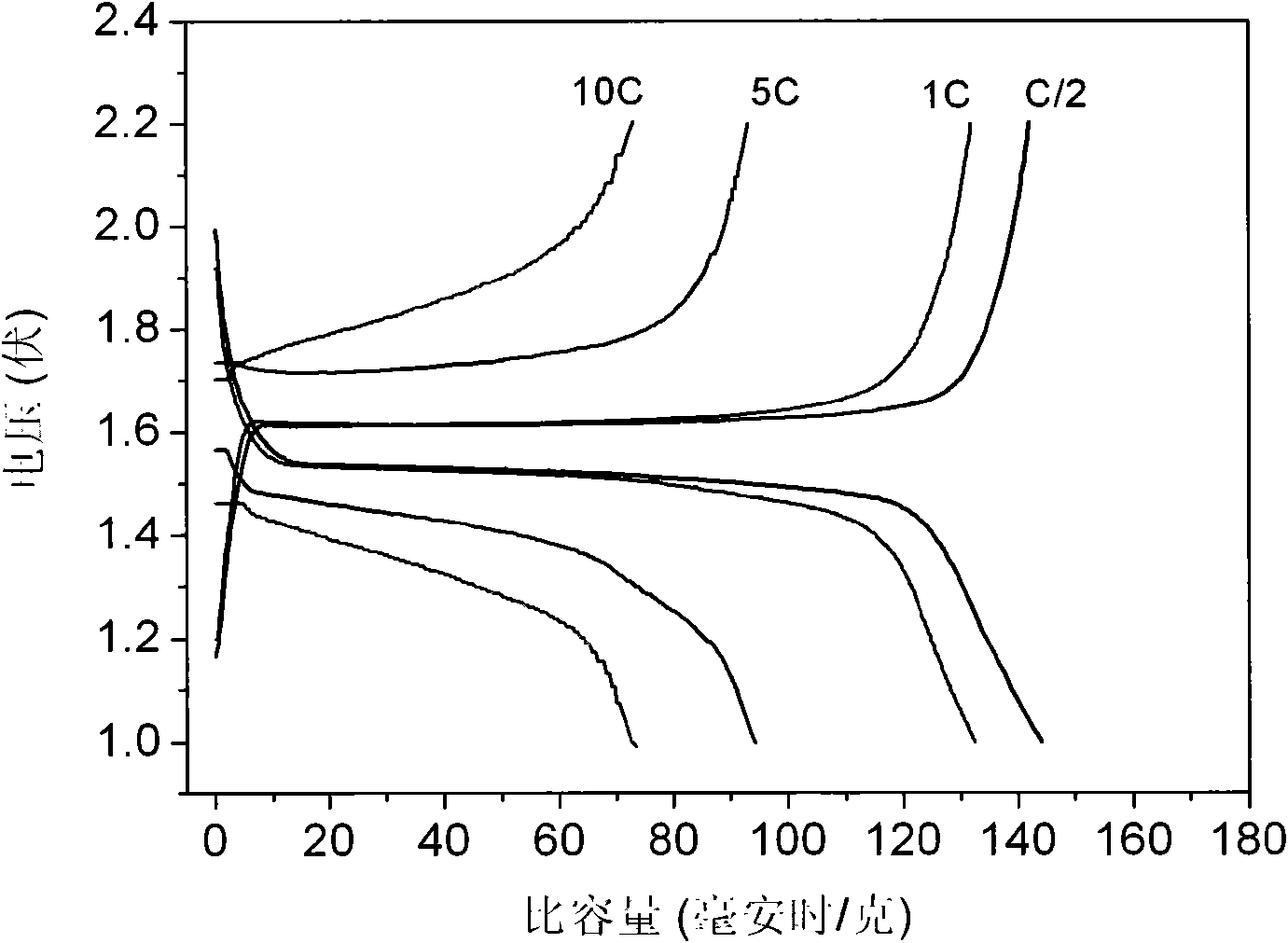
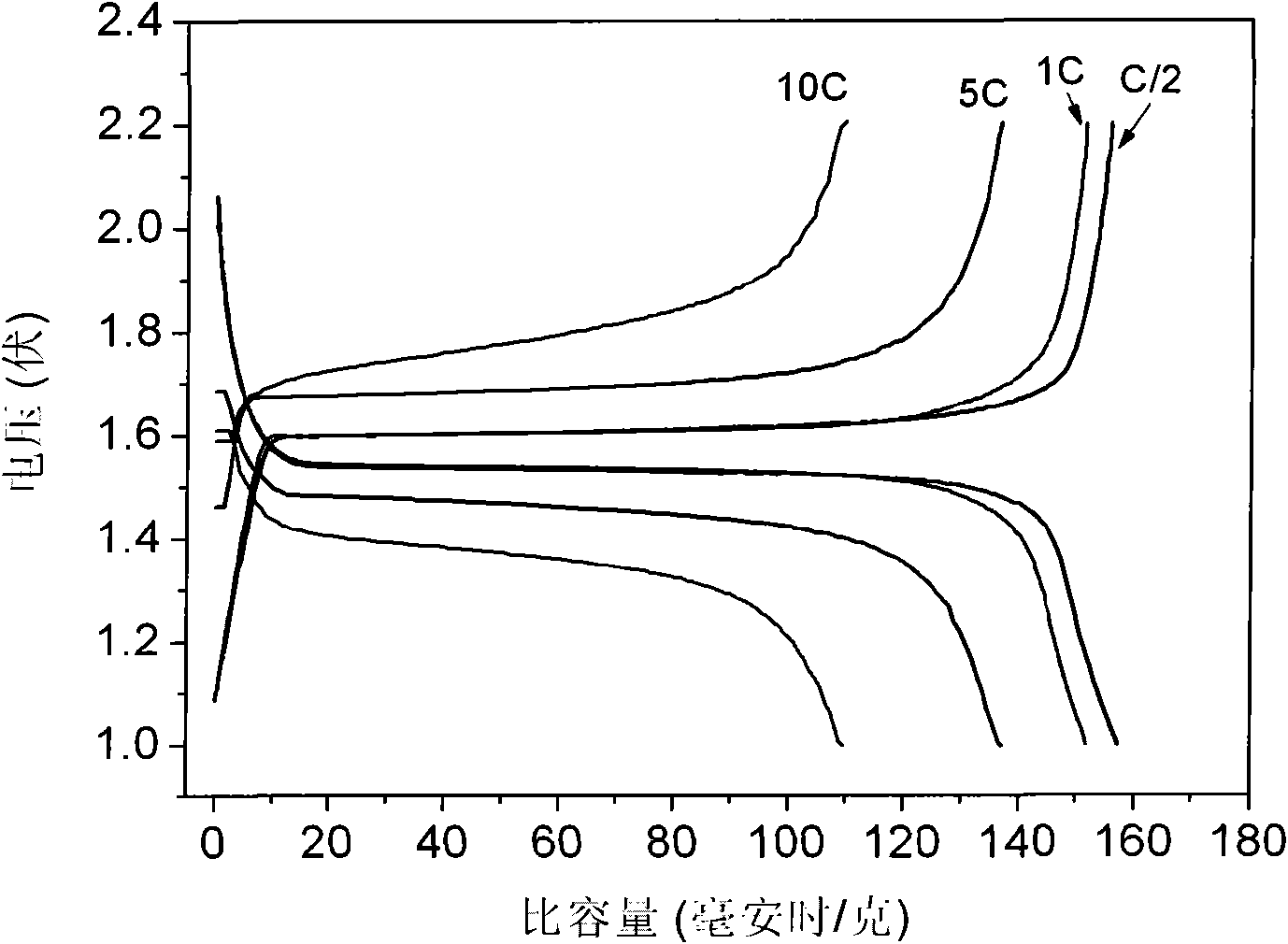
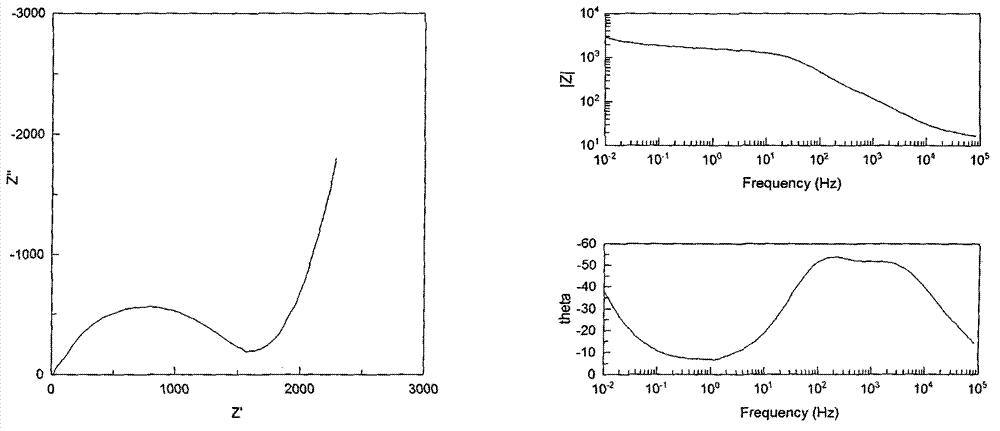
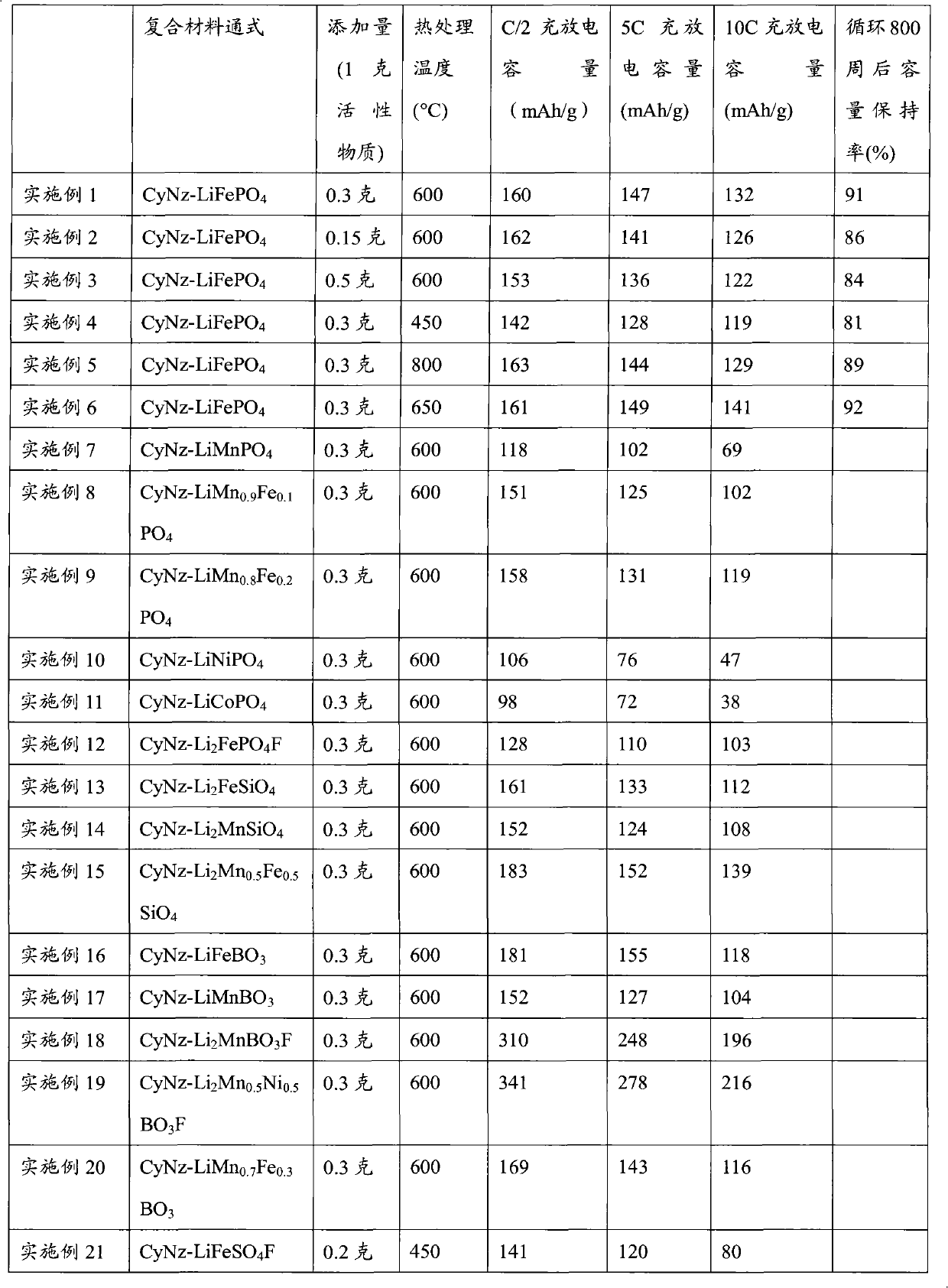
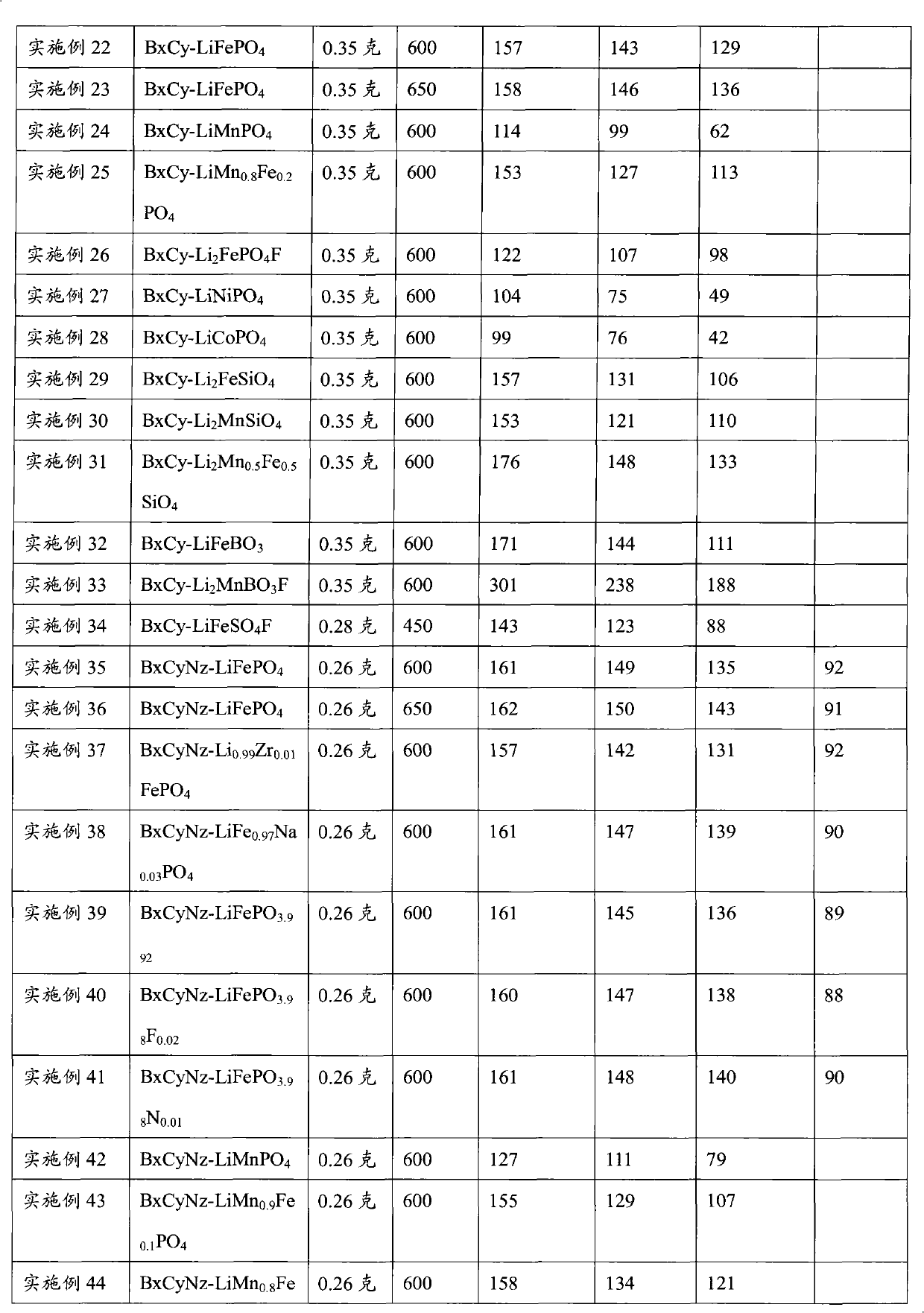
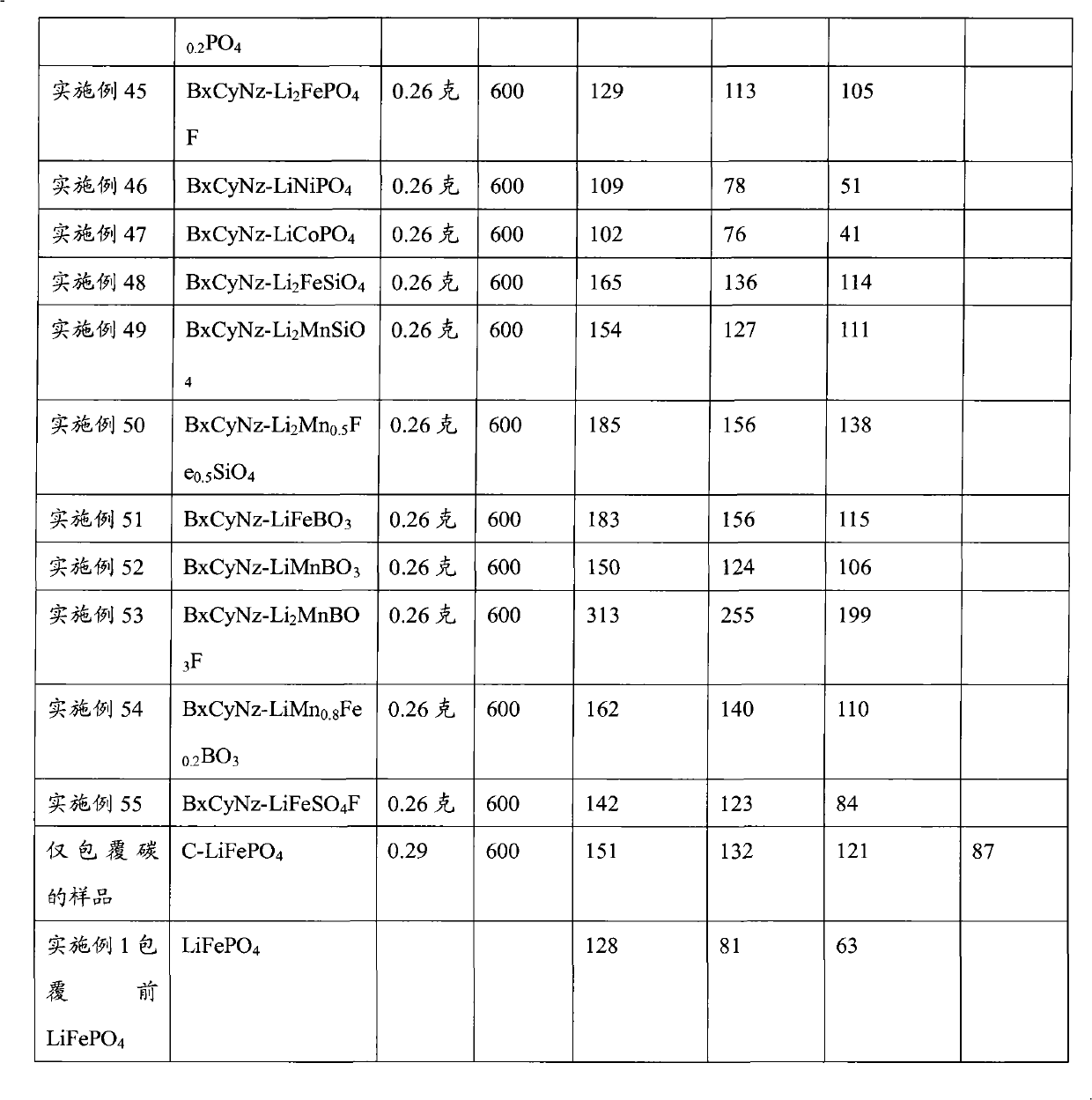
Patents
Literature
30results about How to "Lower activation energy for migration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Spinel composite material, preparation method and application thereof
InactiveCN102315440AImprove electronic conductivityImproved magnification performanceCell electrodesLi-accumulatorsSpinelNitrogen
The invention relates to a spinel composite material, a preparation method and application thereof. The composite material provided by the invention has a general formula of CxNy-(LaM'b)4(McM''d)5O12-eAf, wherein the CxNy is a compound containing carbon and nitrogen. The invention also provides a preparation method and application of the composite material. The invention also provides a cathode comprising the composite material of the invention, and a lithium battery and an electrochemical supercapacitor comprising the cathode. The composition material of the invention has high electronic conductivity and ionic conductivity, particularly high multiplying performance and high cyclical stability.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
Mg<2+>, Al<3+>, Zr<4+> and S<2-> ion co-doped garnet type solid electrolyte
The invention discloses an Mg<2+>, Al<3+>, Zr<4+> and S<2-> ion co-doped garnet type solid electrolyte Li5La3Nb2O12 which is characterized by comprising the stoichiometric equation: Li[5+x+2y+z]La[3-x]MgxAlyZrzNb[2-y-z]O[12-m]Sm, wherein x is equal to 0.1-0.5, y is equal to 0.1-0.2, z is equal to 0.1-0.2, and m is equal to 0.1-0.3; and the solid electrolyte is formed by uniformly mixing Li2CO3, La2O3, MgO, Al2O3, ZrO2, Nb2O5 and thiourea in the molar ratio of (2.7-3.05):(1.25-1.45):(0.1-0.5):(0.05-0.1):(0.1-0.2):(0.8-0.9):(0.1-0.3), and ball milling, pressing and sintering. According to the invention, the lithium-ion conductivity greater than 10<-4>S / cm can be obtained at room temperature.
Owner:NINGBO UNIV
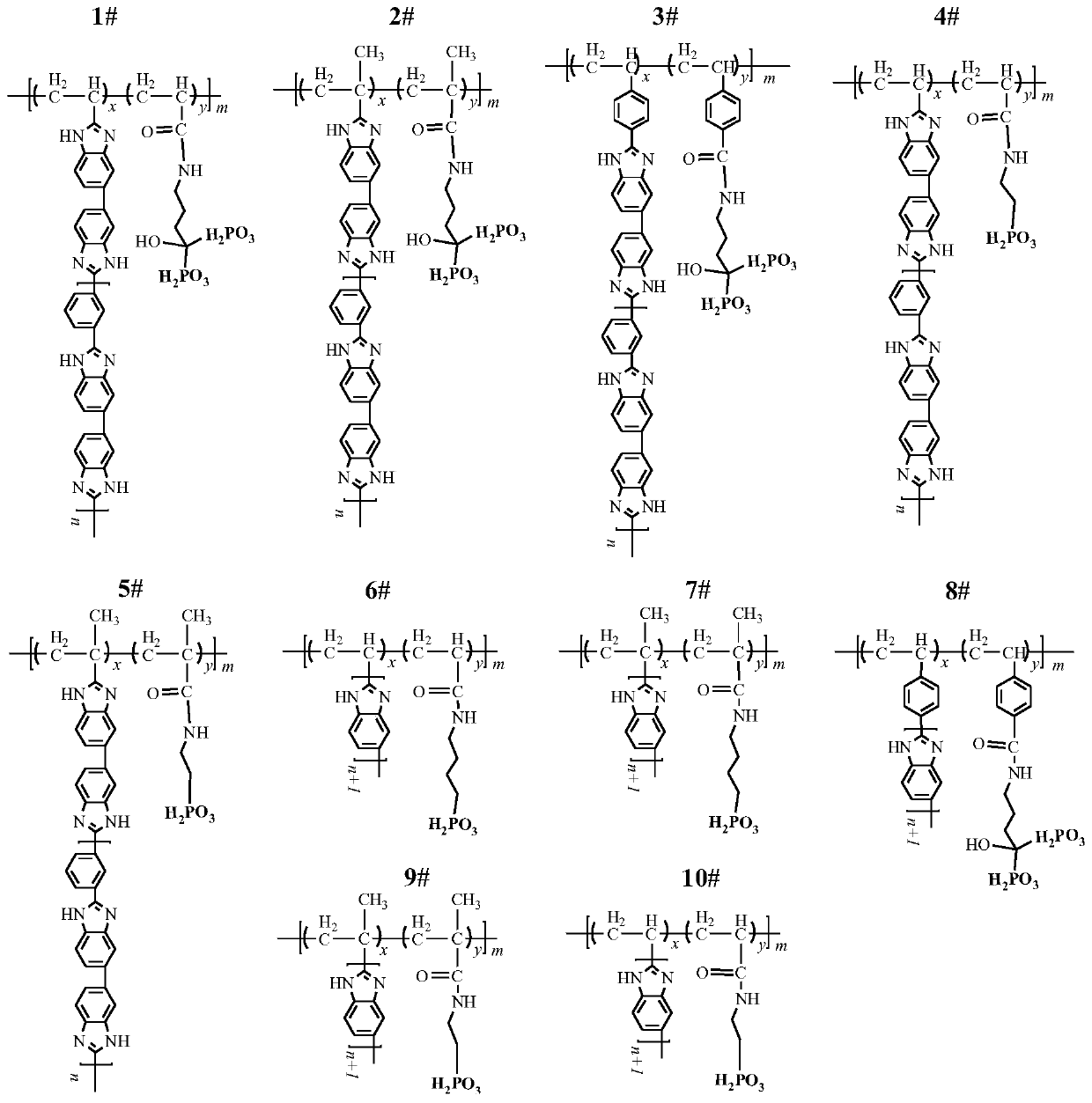
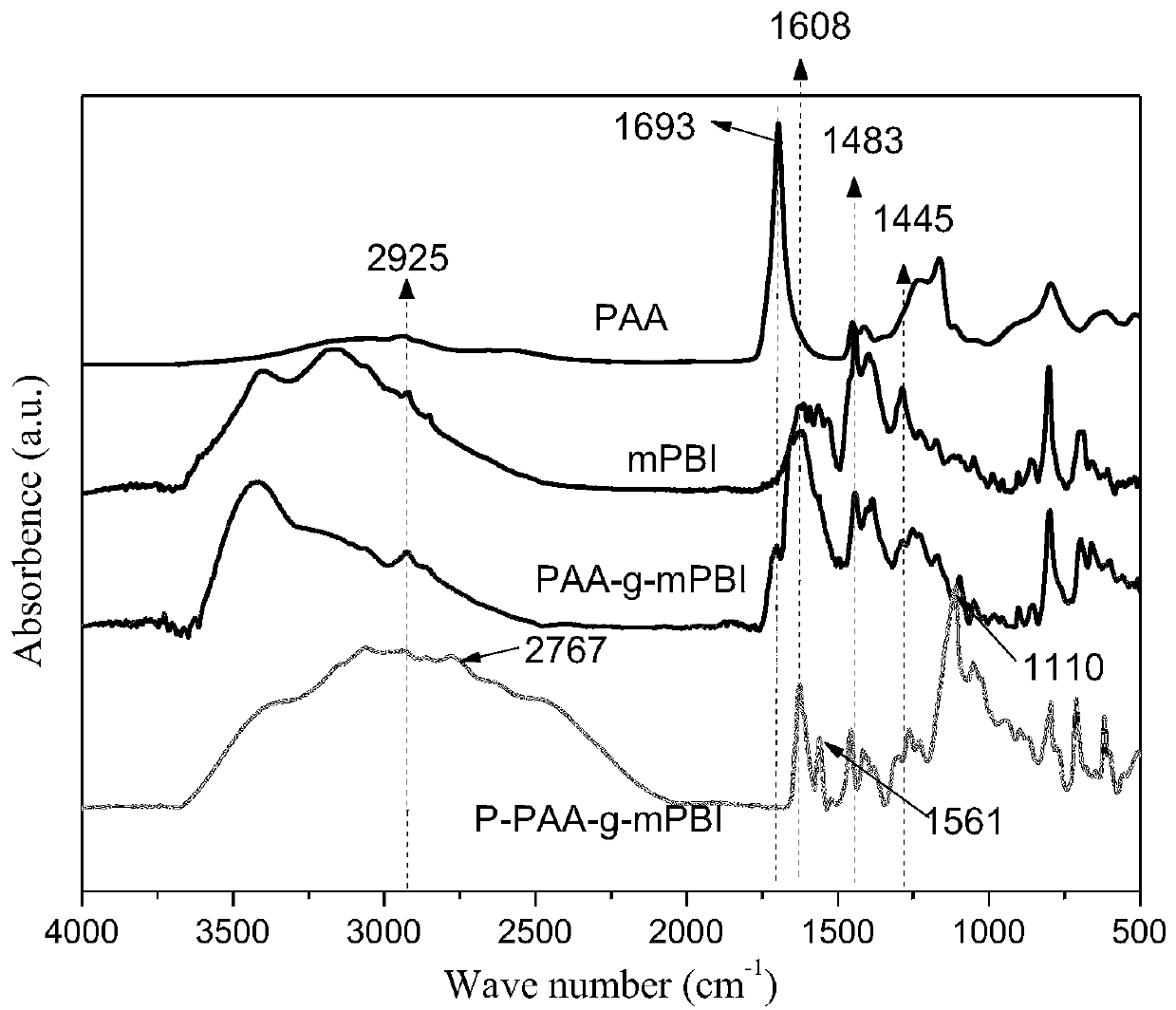
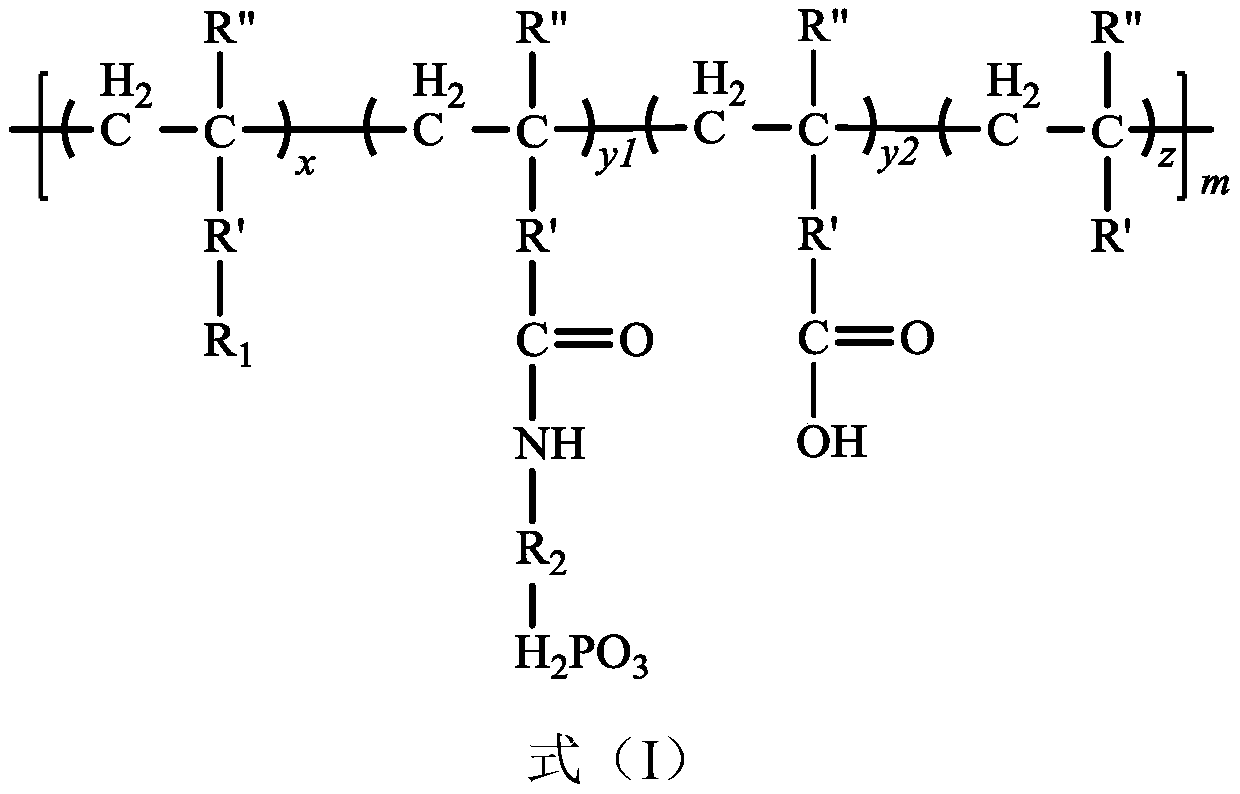
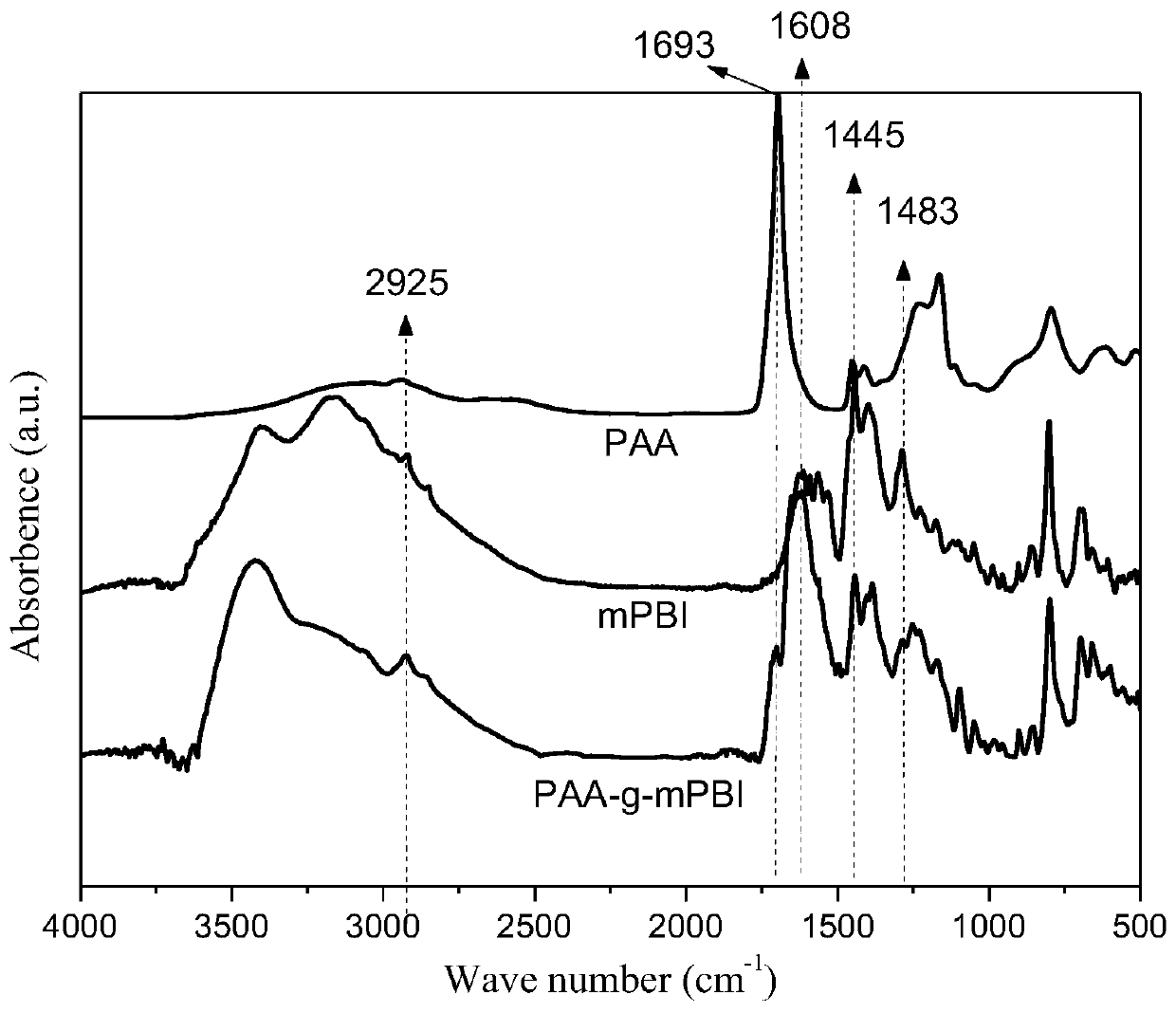
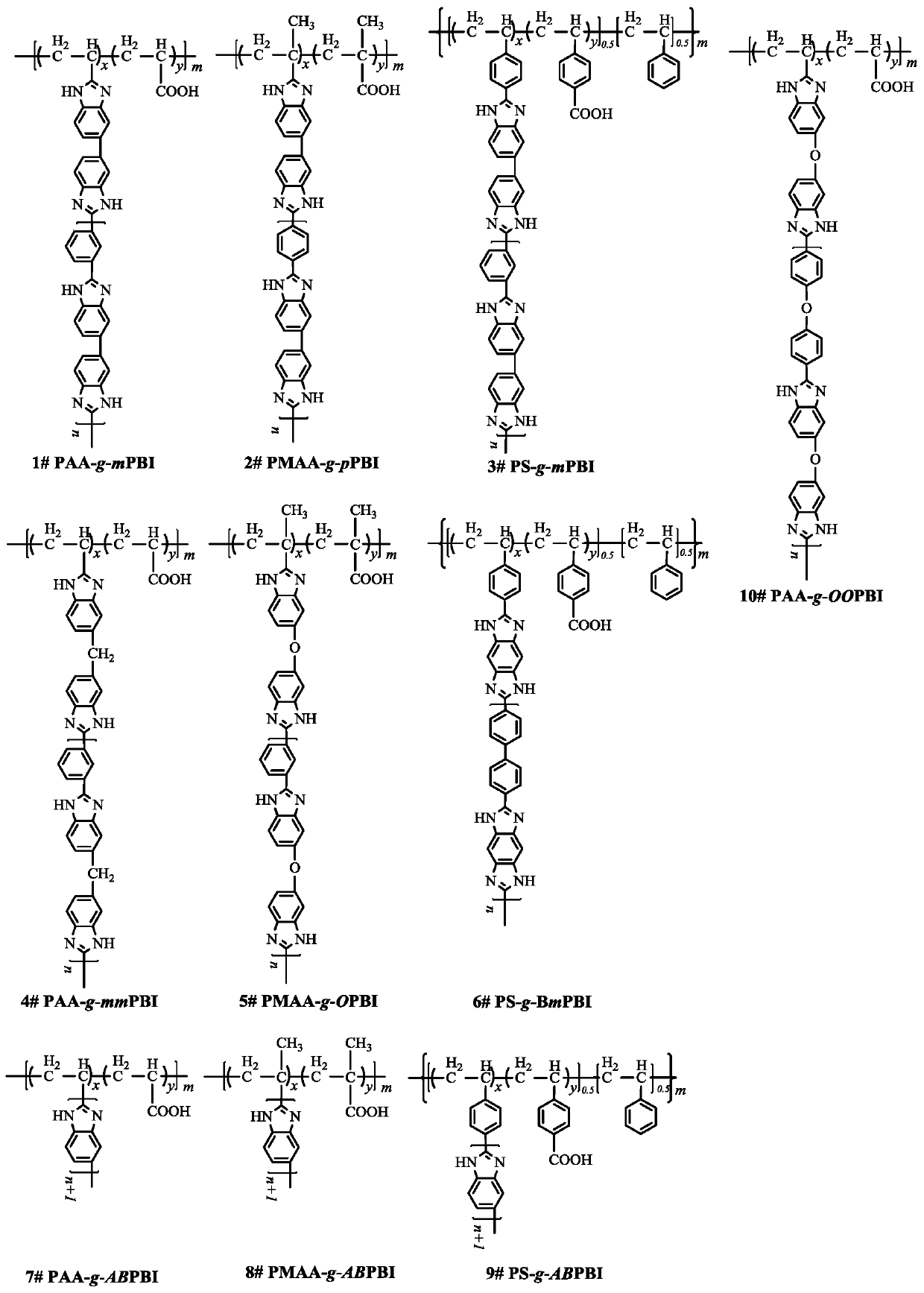
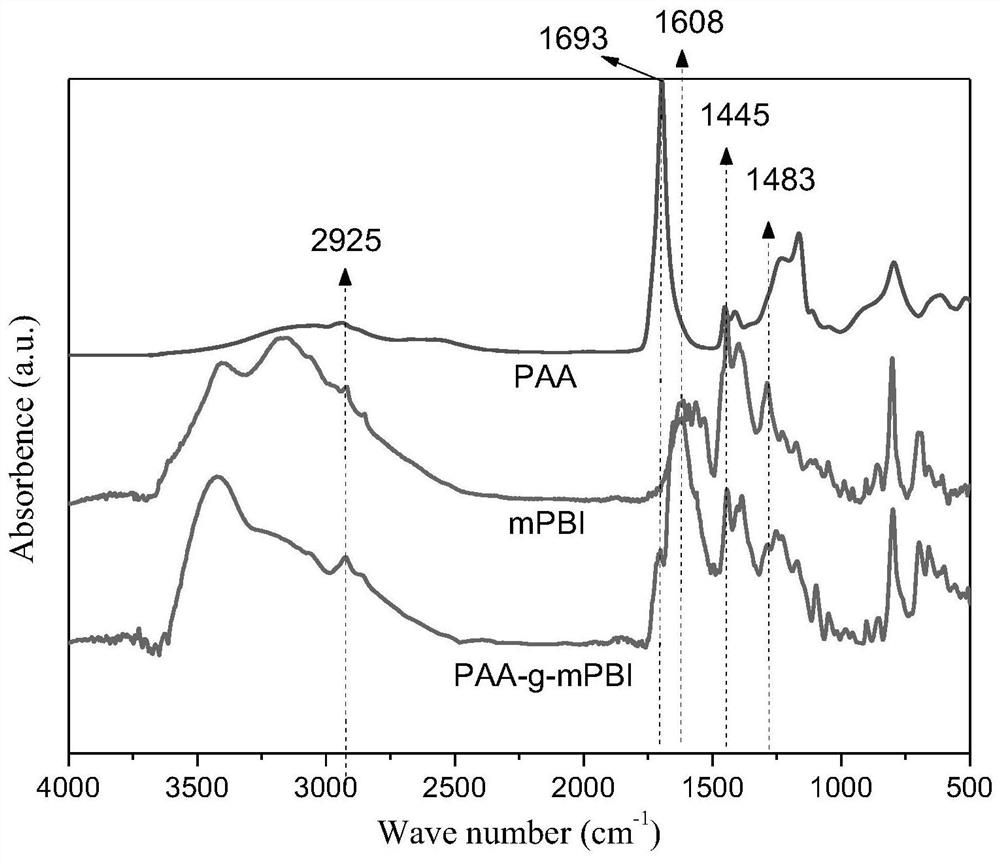
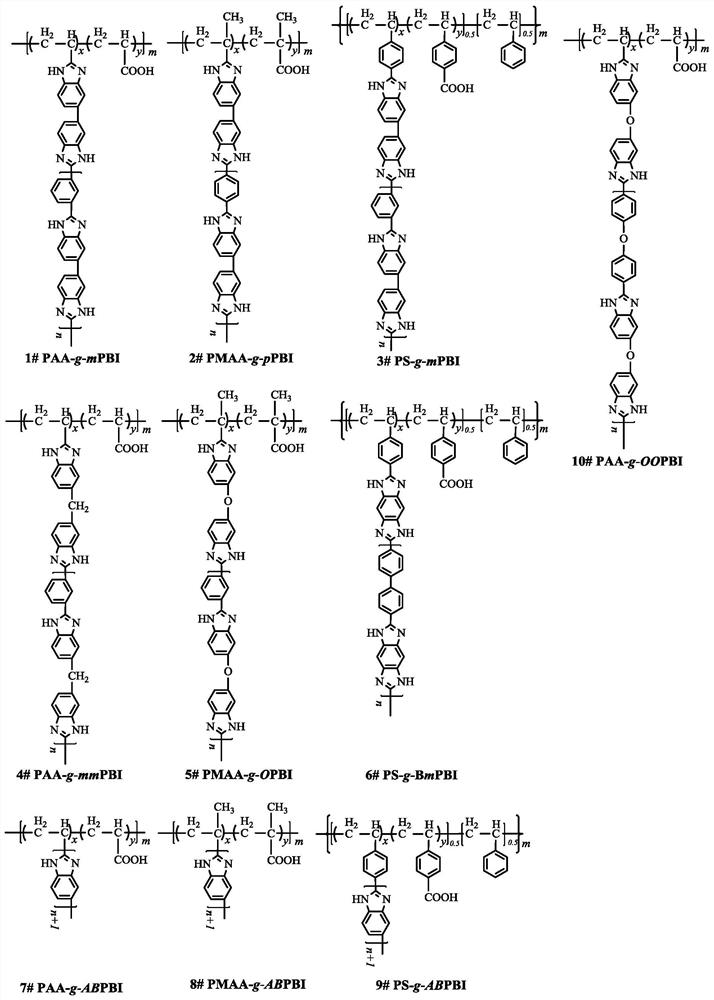
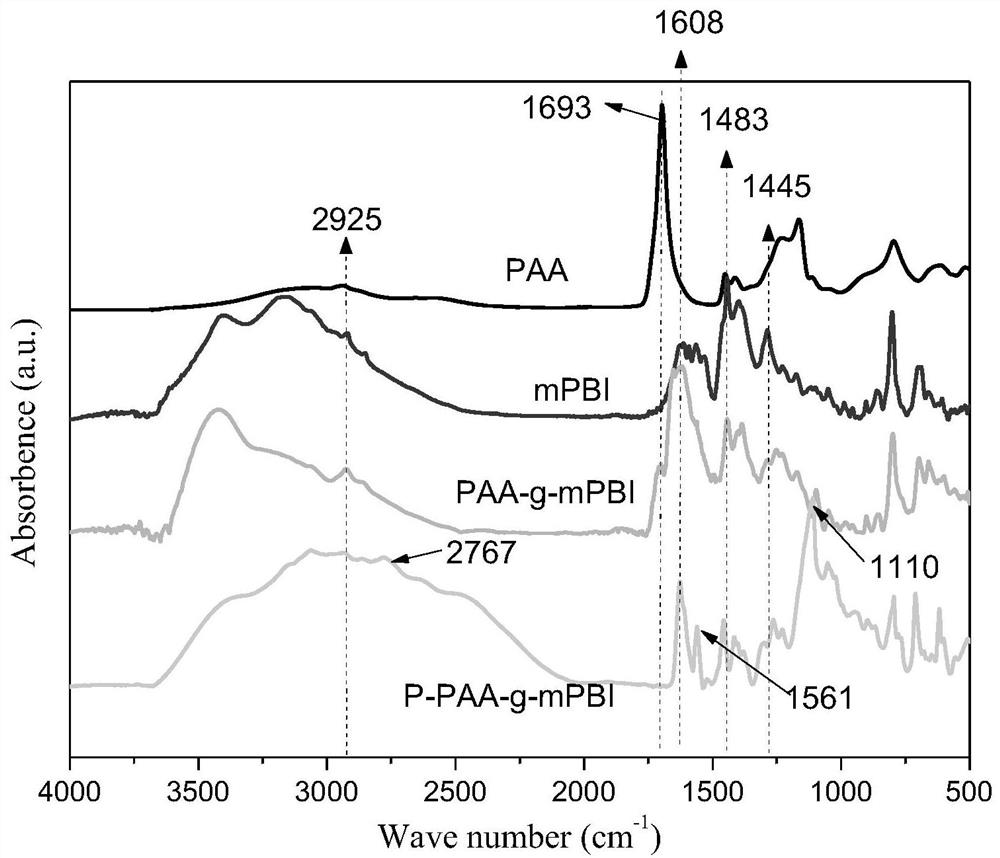
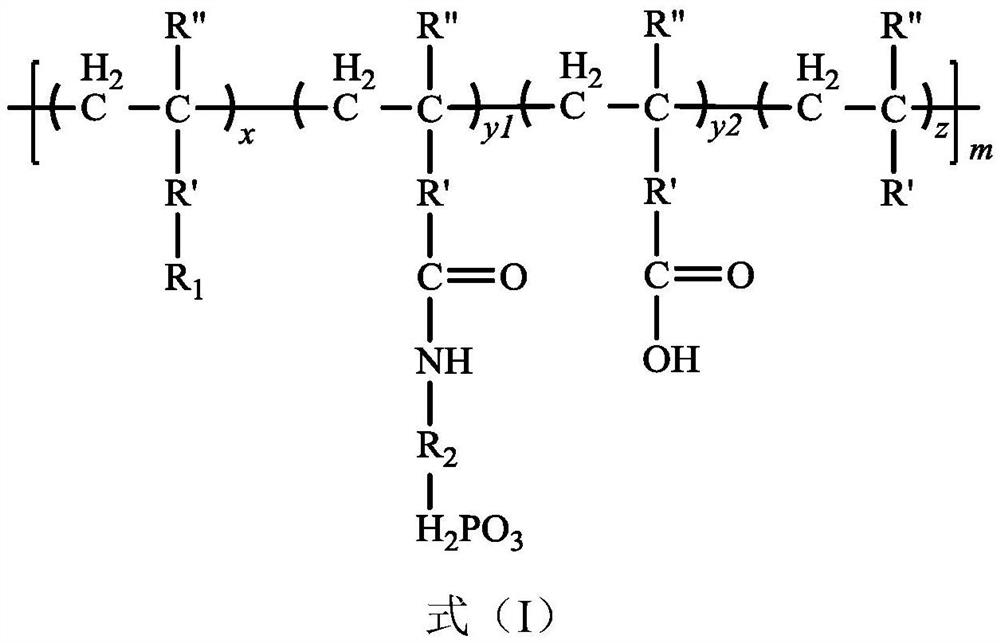
Phosphorylated(polyolefin-g-polybenzimidazole) graft copolymer as well as preparation method and application thereof
ActiveCN110982081ALower activation energy for migrationPromote migrationFuel cellsPolymer sciencePolyolefin
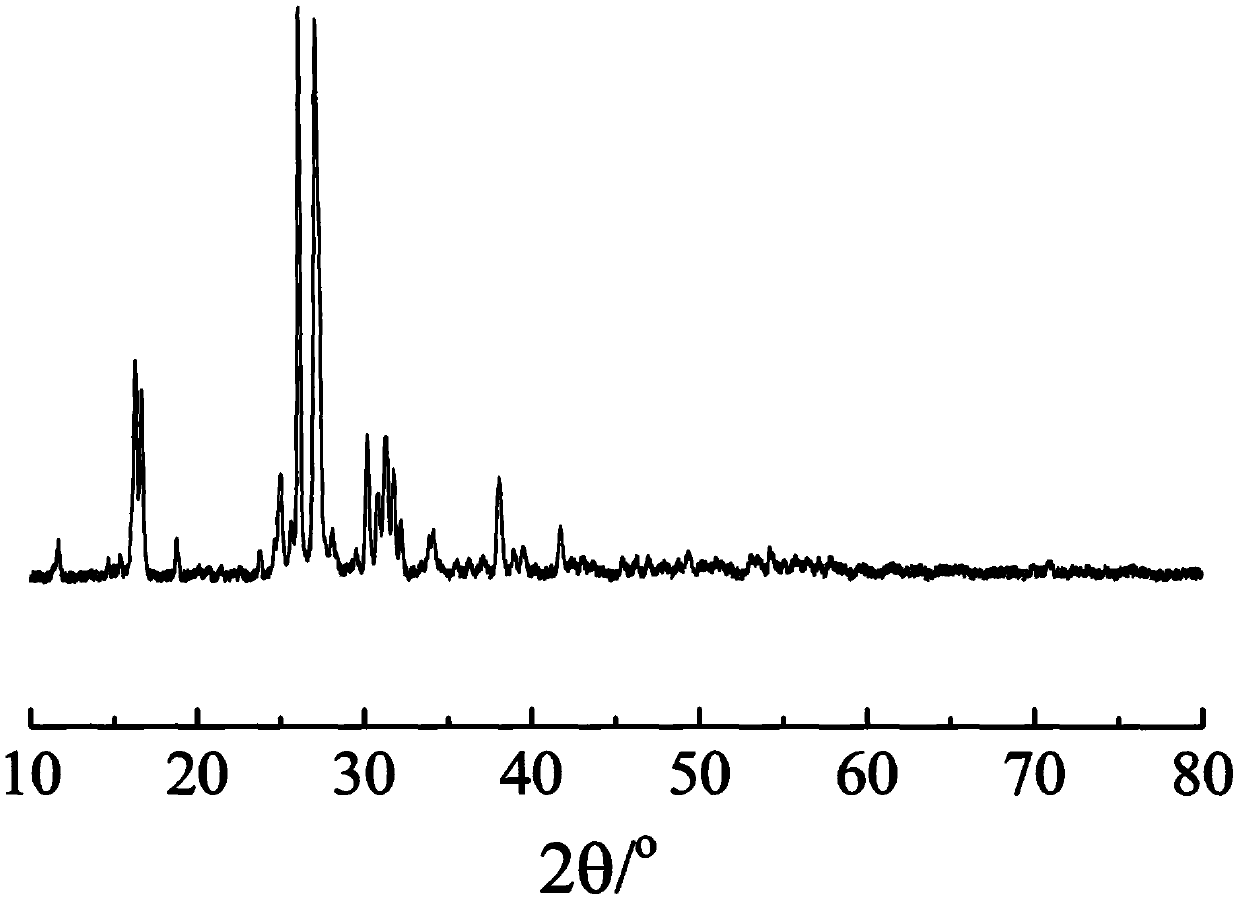
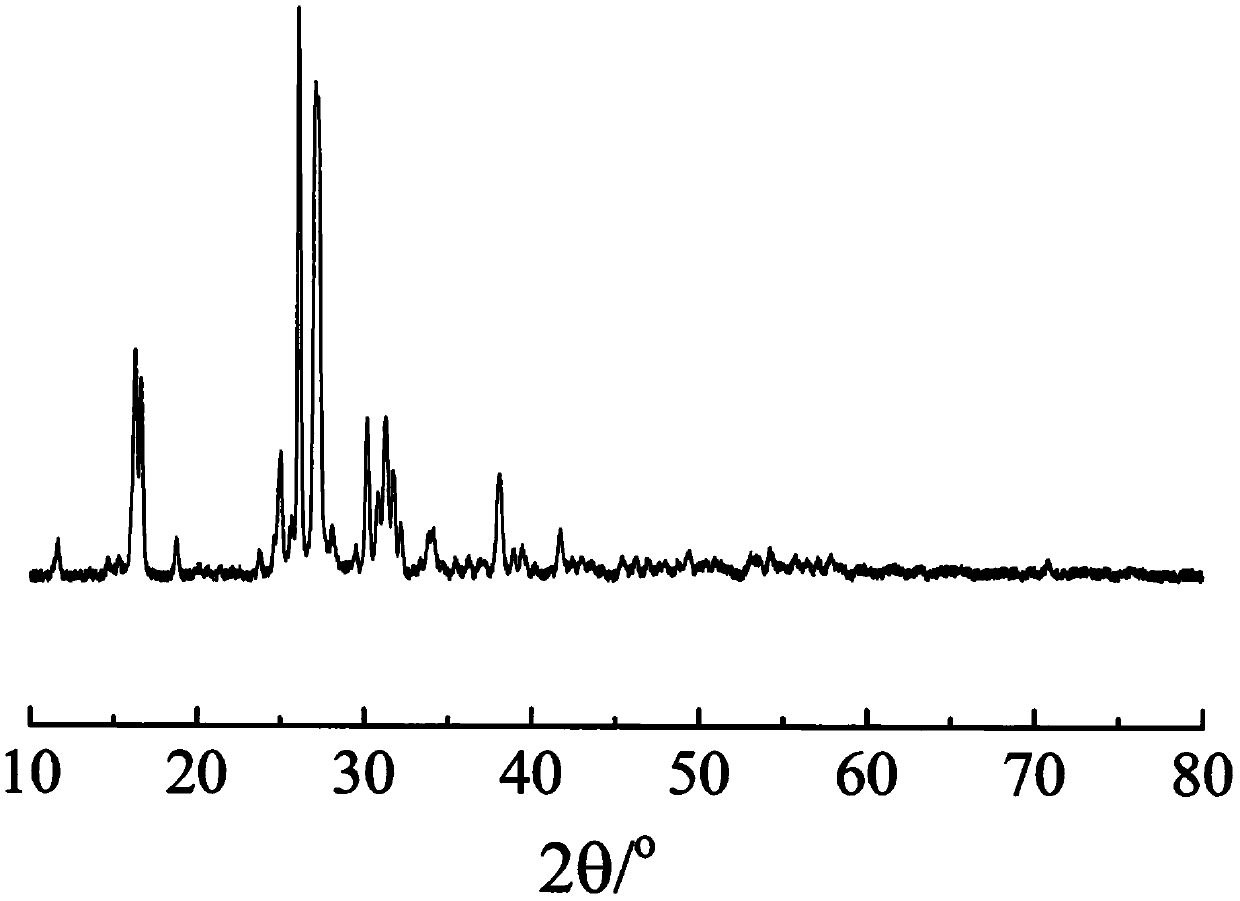
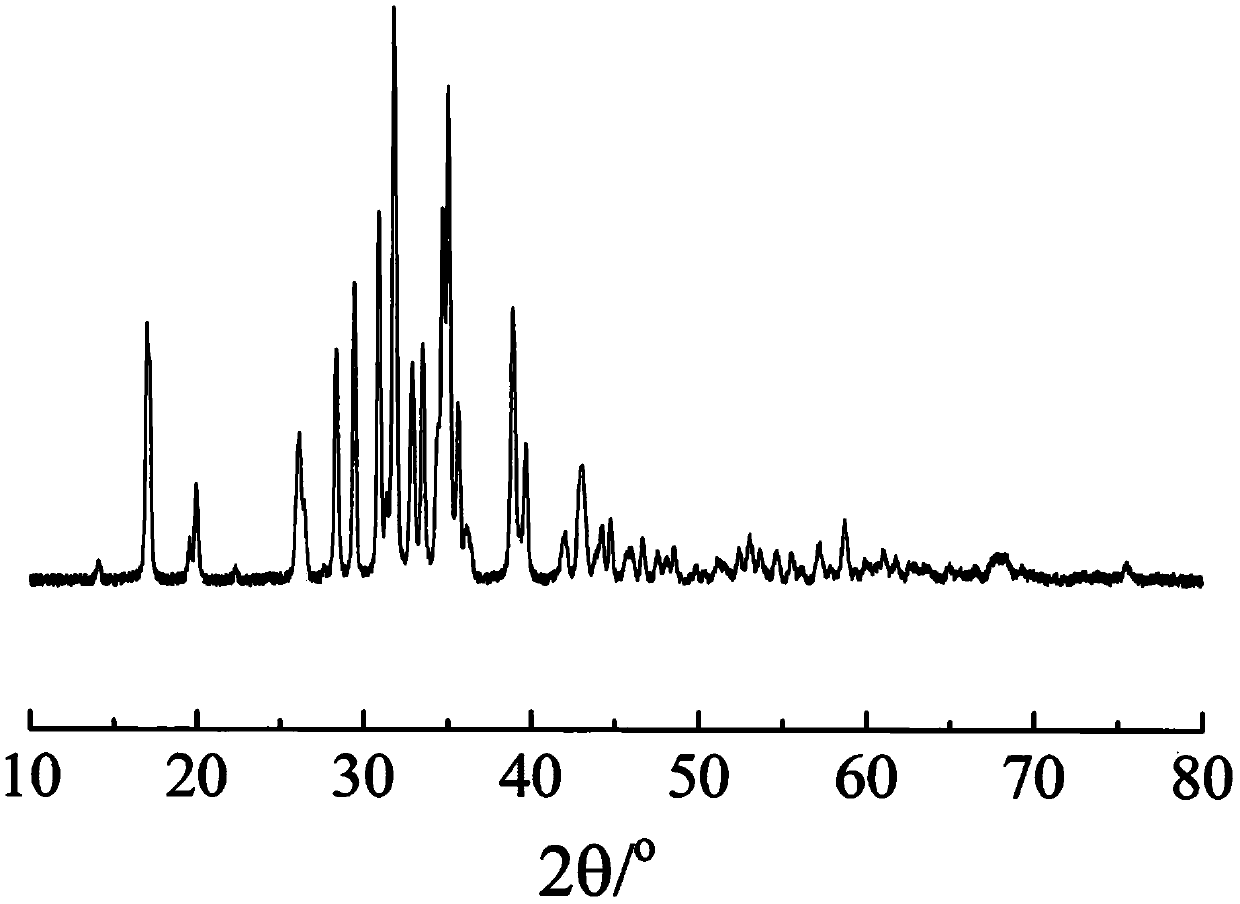
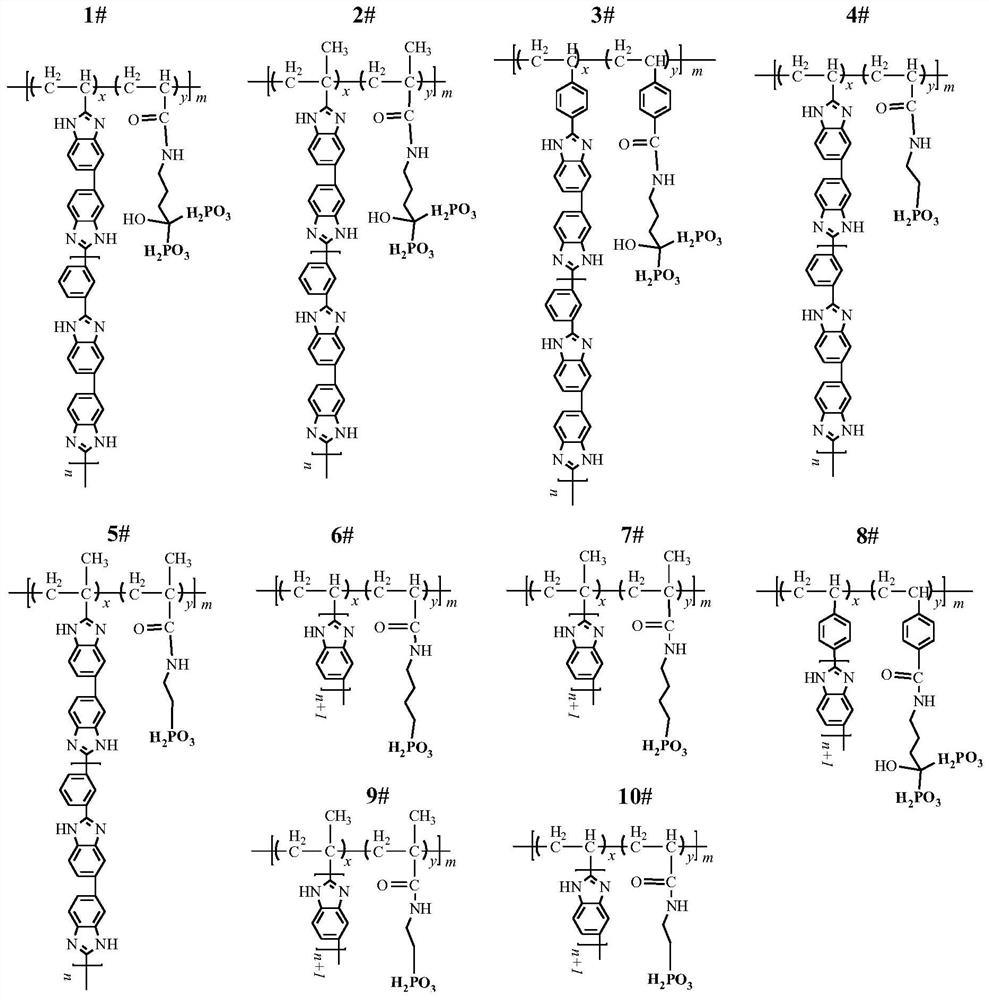
The invention relates to a phosphorylated(polyolefin-g-polybenzimidazole) graft copolymer as well as a preparation method and application thereof. According to the graft copolymer, soft polyolefin isused as a main chain, rigid PBI is used as a branched chain, and the phosphorylated graft copolymer which contains phosphonic acid and has a soft-hard chain segment is further prepared through amino-containing phosphonic acid grafting. The polymers with two properties are subjected to microcosmic phase separation to construct a proton transmission channel, so that the proton conductivity is improved. In addition, the flexible main chain drives the PBI branched chain to move so as to reduce proton migration activation energy, promote migration of phosphoric acid or protons and improve proton conductivity. The grafted amino-containing phosphonic acid can reduce the doping amount of inorganic phosphoric acid, so that the loss of phosphoric acid in the use process is reduced, and the proton conductivity retention rate of the membrane is improved.
Owner:ZHUHAI COSMX BATTERY CO LTD
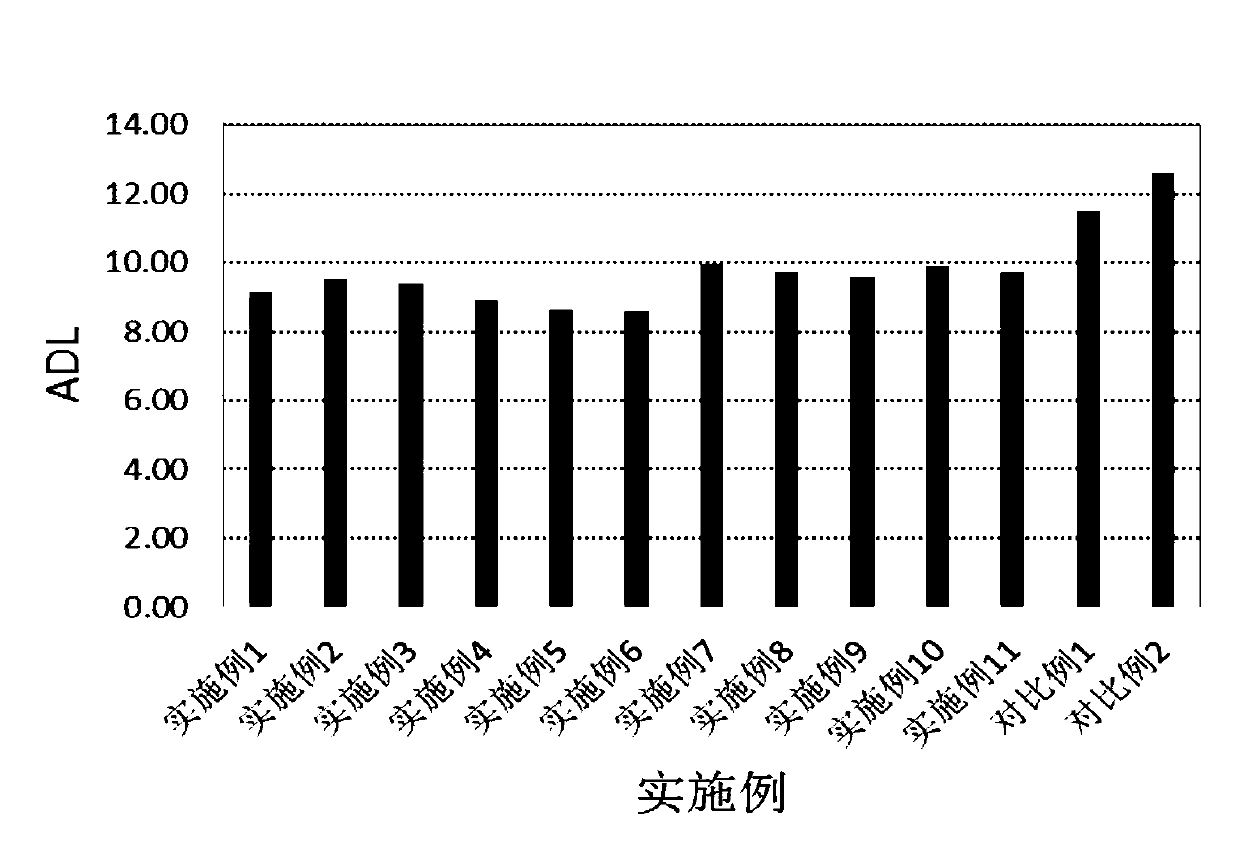
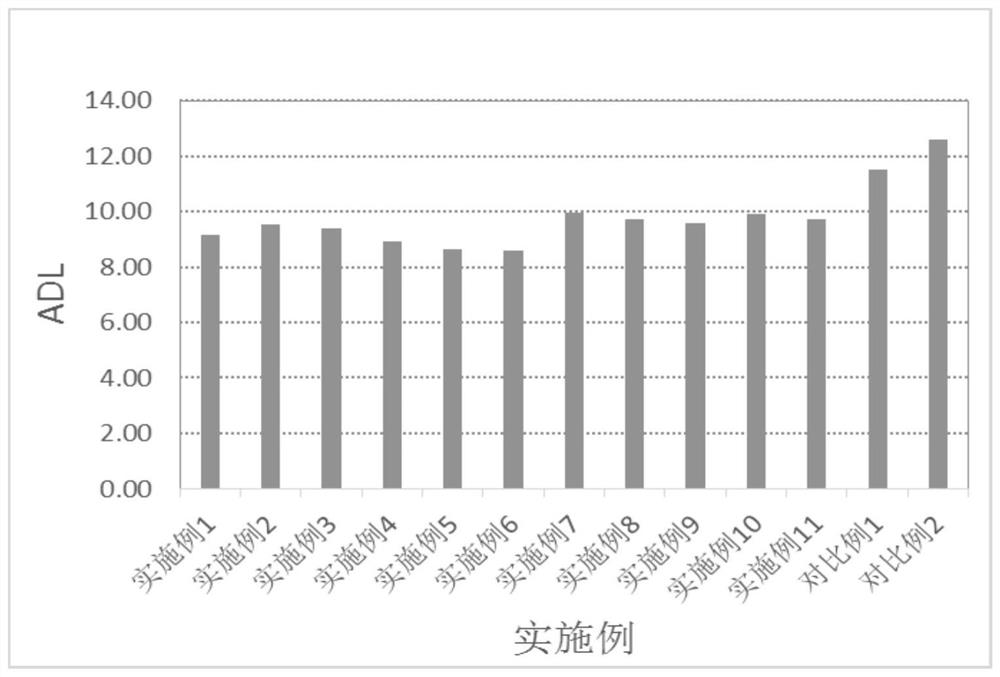
Polyolefin-g-polybenzimidazole grafted copolymer as well as preparation method and application thereof
The invention relates to a polyolefin-g-polybenzimidazole grafted copolymer as well as a preparation method and an application thereof. The grafted copolymer is the grafted copolymer of a polyolefin grafted benzimidazole polymer, which is obtained by carrying out a condensation reaction on a terminal amino group in the benzimidazole polymer and a carboxyl group in an olefin polymer with the side chain containing the carboxyl group. According to the present invention, a proton transmission channel is constructed through a phase separation structure of the two chain segments so as to improve theproton conductivity, such that the high temperature proton exchange membrane with the high proton conductivity can be obtained under the low phosphoric acid doping level (ADL is less than 10) condition, and the preparation method is simple, is simple to operate, and can be used for fuel cells, flow cells and the like.
Owner:ZHUHAI COSMX BATTERY CO LTD
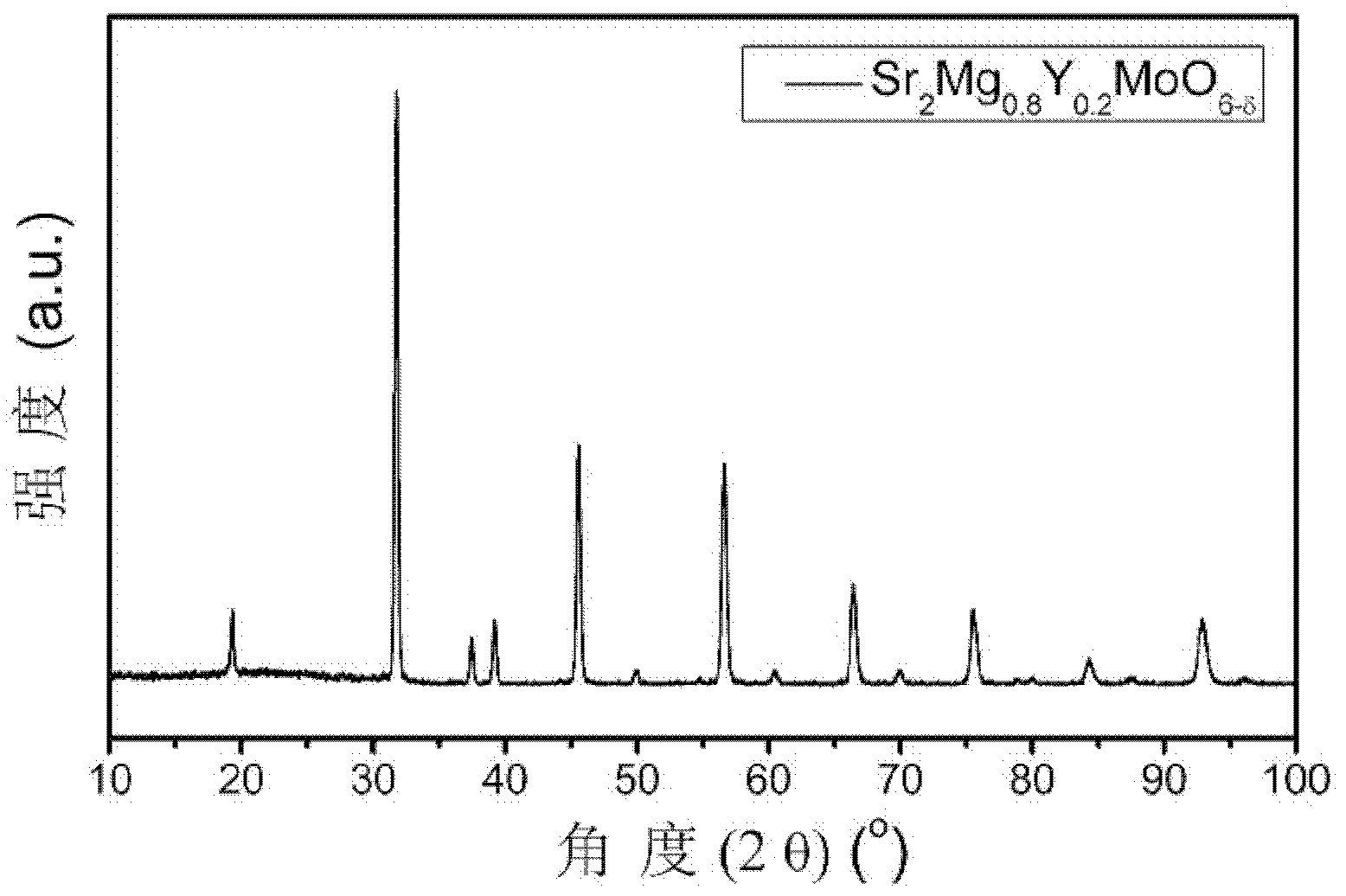
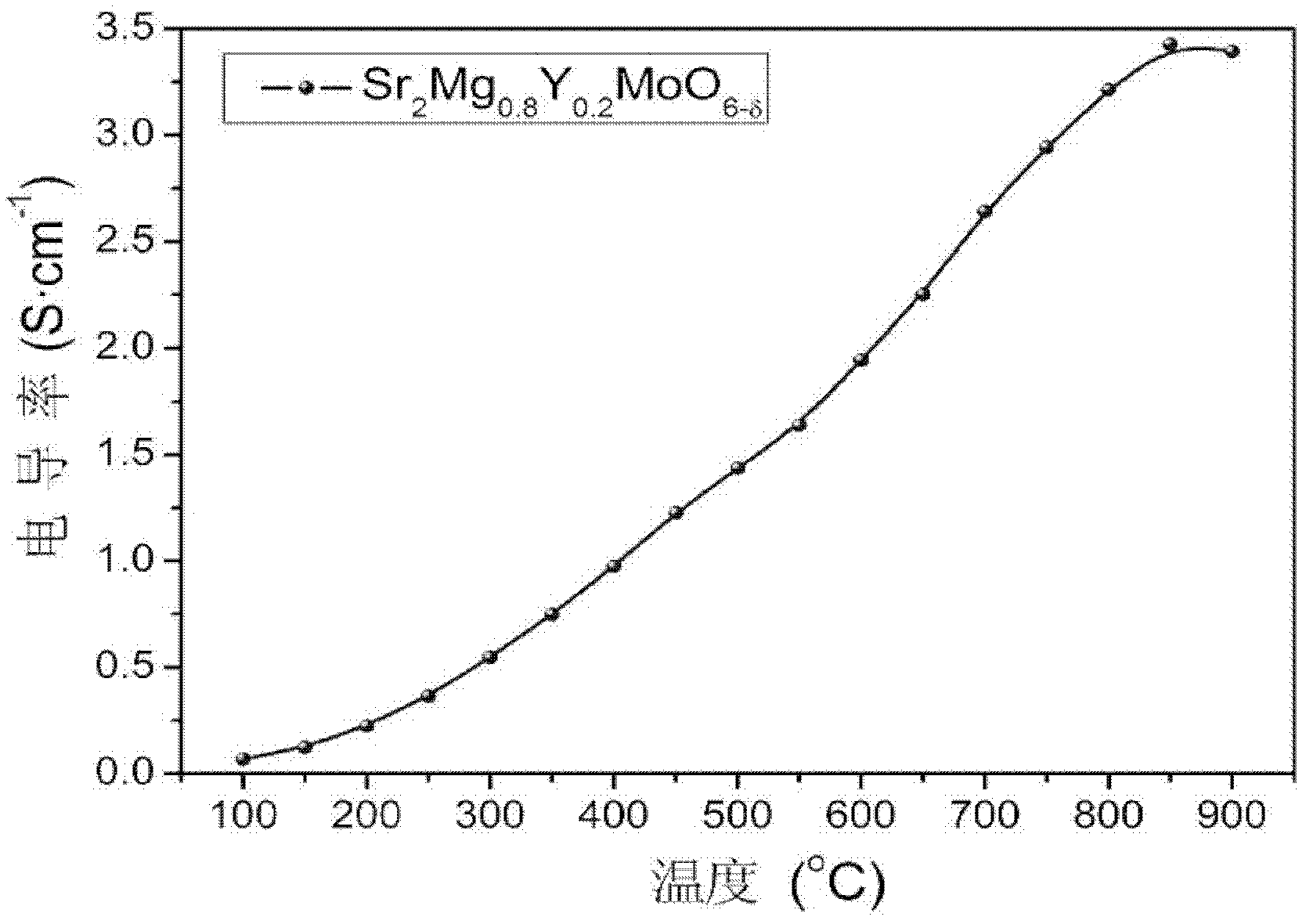
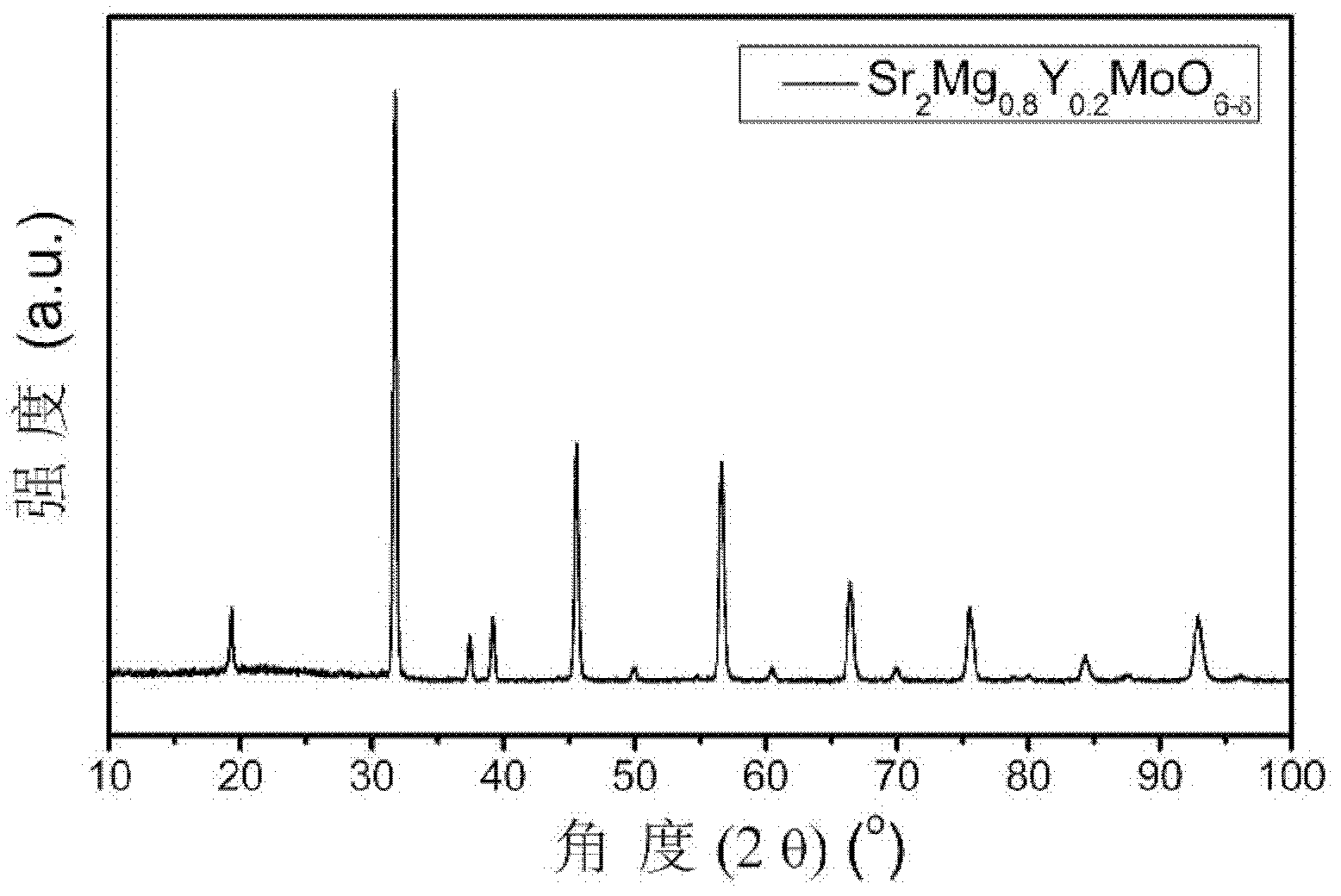
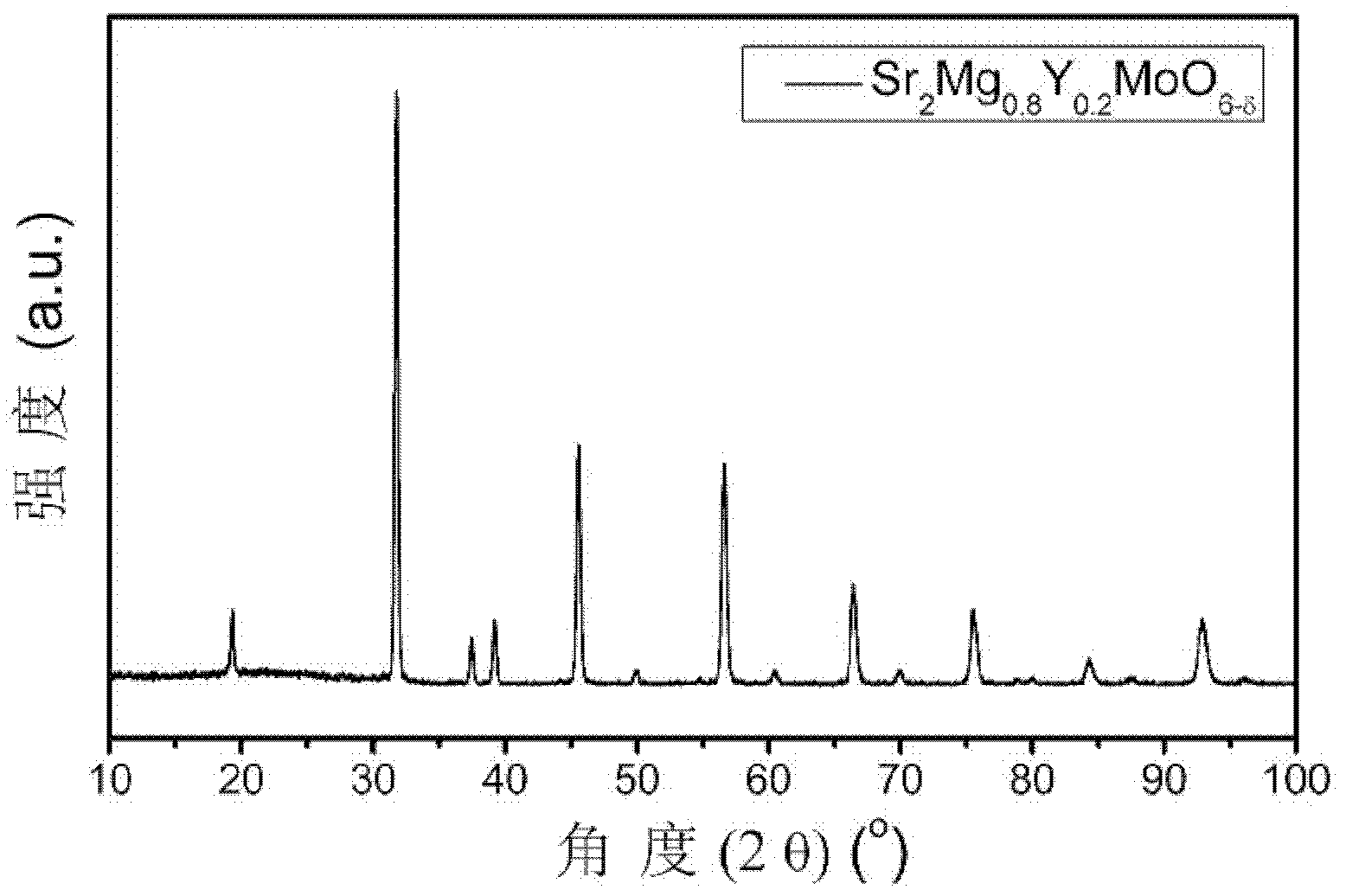
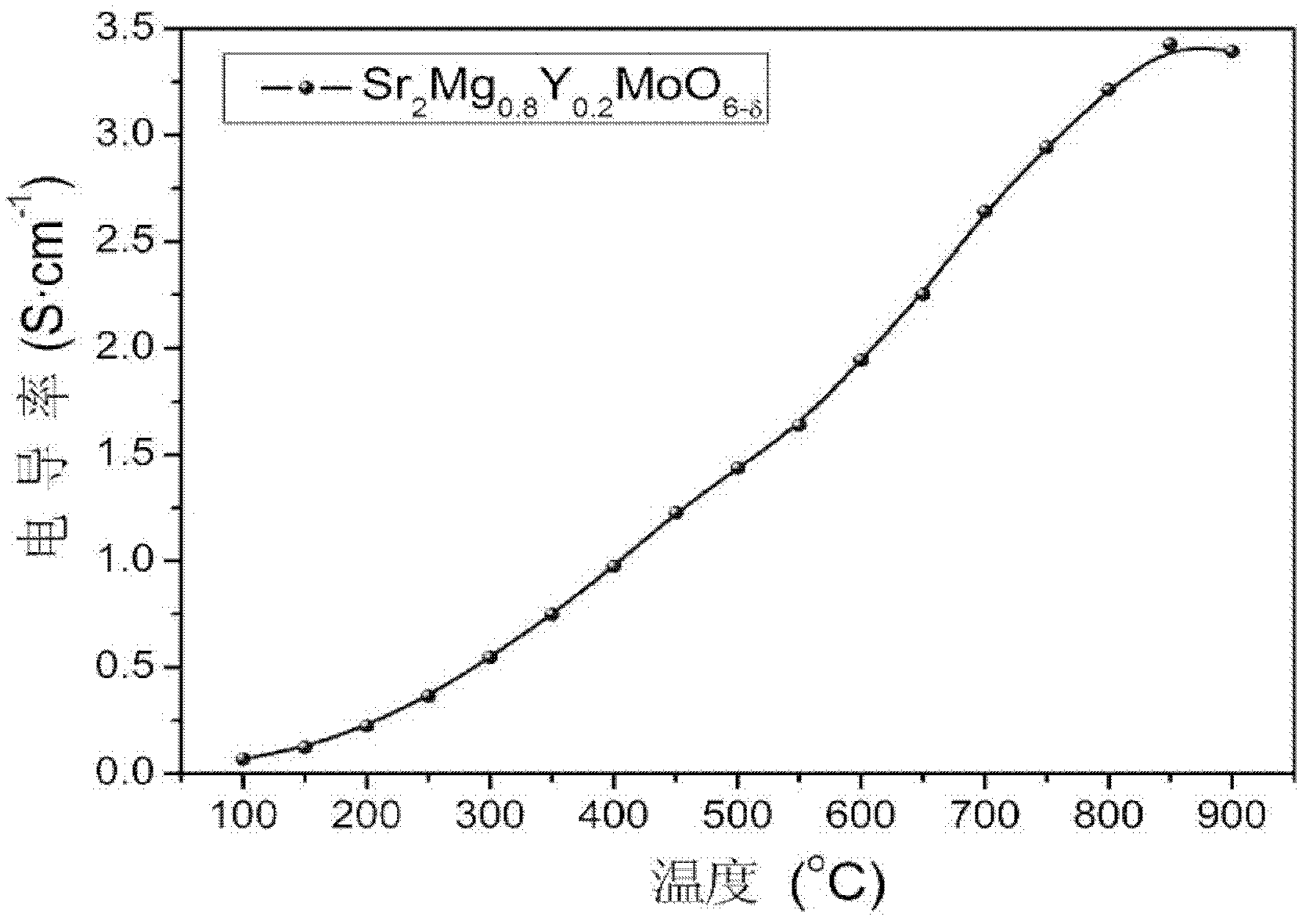
High-conductivity double-perovskite-type anode material and preparation method thereof
ActiveCN102593467AIncrease concentrationReduced migration activationCell electrodesElectrical conductorFuel cells
The invention discloses a high-conductivity double-perovskite-type anode material and a preparation method thereof and belongs to the field of solid oxide fuel cells. The high-conductivity double-perovskite-type anode material is characterized in that a B site of a double-perovskite-type (A2BB'O6) solid oxide fuel cell anode material Sr2MgMoO6 is doped with Y so that the high-conductivity double-perovskite-type anode material which is a mixed conductor having a double-perovskite structure is obtained. The preparation method provided by the invention comprises the following steps of pressing B site-doped Sr2Mg1-xYxMoO6 powder (x is in a range of 0.1 to 0.2) into a sample strip under certain pressure, carrying out sintering at a high temperature in an air atmosphere, carrying out reduction under reduction conditions, and carrying out conductivity measuring, wherein compared with a conductivity measured before doping, a conductivity measured after doping is improved 5.8 times (x=0.2). Through the preparation method provided by the invention, the high-conductivity double-perovskite-type anode material which is a porous film-type anode material Sr2Mg1-xYxMoO6 (x is in a range of 0.1 to 0.2) is obtained, and has good combinability and good chemical compatibility with electrolytes of GDC and LSGM, and carbon distribution-resistant and sulfur poisoning-resistant capabilities higher than carbon distribution-resistant and sulfur poisoning-resistant capabilites of the traditional anode material.
Owner:UNIV OF SCI & TECH BEIJING
A mg2+, al3+, zr4+, f- ion co-doped garnet-type solid electrolyte
InactiveCN102780031BImprove conductivityModerately expanded sectionSecondary cellsLithiumPhysical chemistry
Owner:NINGBO UNIV
Electric field induced crystallization K<6.15>Zn<0.05>B<0.2>Al<0.1>P<0.05>Zr<0.05>Si<1.6>O<7> potassium fast ion conductor and preparation method thereof
InactiveCN110372348ALower activation energy for migrationFacilitate conductionSolid electrolytesSecondary cellsHigh concentrationElectrical conductor
The invention discloses an electric field induced crystallization K<6.15>Zn<0.05>B<0.2>Al<0.1>P<0.05>Zr<0.05>Si<1.6>O<7> potassium fast ion conductor and a preparation method thereof. Al<3+> and B<3+>are used for partially substituting Si<4+> ions, high-concentration interstitial potassium ions are generated in a crystal, and the reduction of the migration activation energy of potassium ions is facilitated; the electron conductivity of the fast ion conductor is further reduced by doping P<5+>; the size of migration channels of the potassium ions is adjusted by doping B<3+> with a small ionicradius to adapt to the rapid migration of the potassium ions; Zr<4+> is partially doped to form a distorted lattice structure to increase lattice imperfection to facilitate potassium ion conduction; cation vacancies are generated by doping Zn<2+> to increase migratory routes of the potassium ions; and the surface of K6Si2O7 particles is modified during the preparation to form an easy-sintering property. Meanwhile, the introduction of a strong direct current electric field induces crystallization to accelerate the crystallization rate, lower the crystallization temperature and increase the crystal integrity. The synergistic effects enable normal temperature potassium ion conductivity of the potassium fast ion conductor to exceed 5*10<-4> S / cm and to be closer to the potassium ion conductivity of a liquid electrolyte.
Owner:NINGBO UNIV
Fe2O3/FeF3-2xOx/Fe<3+>,Ce<4+> doped zirconium fluoride layer structure positive electrode material of lithium battery and preparation method thereof
InactiveCN105914350AImprove electrochemical performancePromote sheddingCell electrodesSecondary cellsPhysical chemistryFluoride
The invention provides a Fe2O3 / FeF3-2xOx / Fe<3+>,Ce<4+> doped zirconium fluoride layer structure positive electrode material of a lithium battery and a preparation method thereof. According to the method, Fe<3+>,Ce<4+> doped zirconium fluoride is prepared through solid-phase synthesis; then on the basis of the characteristic that FeF3 is prone to gradual oxidation into Fe2O3 at a high temperature, FeF3-2xOx (wherein 0<x<0.3) and a Fe2O3 layer successively coat Fe<3+>,Ce<4+> doped zirconium fluoride particles so as to improve surface electron conduction capability of Fe<3+>,Ce<4+> doped zirconium fluoride and to resist harmful effect of an organic electrolyte on particle surfaces; and comprehensive electrochemical performance of zirconium fluoride is greatly improved through Fe<3+>,Ce<4+> doping.
Owner:NINGBO UNIV
Polyanion composite material, its preparation method and application
ActiveCN102339999BImprove electronic conductivityImproved magnification performanceCell electrodesSecondary cellsNitrogenBoron
The invention relates to a polyanion composite material, which has a general formula of BxCyNz-LiaD' bMcD'' dXOeAf, with BxCyNz as a compound of boron and carbon, or carbon and nitrogen, or boron, carbon and nitrogen. The invention provides a preparation method of the composite material and its application. The invention also provides a positive electrode with the composite material of the invention and a lithium battery containing the positive electrode. The composite material provided in the invention has high electronic conductivity and ionic conductivity, especially has an excellent rate performance and good cycle stability.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
K6Si2O7 potassium fast ion conductor synergistically doped with P<5+>, Al<3+> and Be<2+> ions and preparation method of K6Si2O7 potassium fast ion conductor
InactiveCN110372349ALower activation energy for migrationLow electronic conductivitySolid electrolytesSecondary cellsElectrical conductorIonic conductance
The invention relates to a K6Si2O7 potassium fast ion conductor synergistically doped with P<5+>, Al<3+> and Be<2+> ions and a preparation method of the K6Si2O7 potassium fast ion conductor. The K6Si2O7 potassium fast ion conductor is characterized in that the stoichiometric formula is K<6+2x+y-z>Be<x>Al<y>P<z>Si<2-x-y-z>O7, wherein <x>=0.05-0.10; <y>=0.05-0.10; <z>=0.02-0.05; and the normal temperature potassium ion conductivity exceeds 5*10<-4> S / cm. Al<3+> and Be<2+> are adopted to partially substitute for Si<4+> ions, and interstitial potassium ions are generated in crystals to reduce themigration activation energy of the potassium ions; the electronic conductivity of the fast ion conductor is further reduced through P<5+> doping; the size of migration channels of the potassium ions is adjusted through the doping of Be<2+> with a small ionic radius to adapt to the rapid migration of the potassium ions; and the surface of K6Si2O7 particles is modified in the preparation process toform the easy sintering characteristic. The synergistic effects enable the normal temperature potassium ion conductivity of the potassium fast ion conductor to exceed 5*10<-4> S / cm and be closer to the potassium ion conductivity of a liquid electrolyte.
Owner:NINGBO UNIV
High-conductivity double-perovskite-type anode material and preparation method thereof
ActiveCN102593467BIncrease concentrationReduced migration activationCell electrodesElectrical conductorFuel cells
The invention discloses a high-conductivity double-perovskite-type anode material and a preparation method thereof and belongs to the field of solid oxide fuel cells. The high-conductivity double-perovskite-type anode material is characterized in that a B site of a double-perovskite-type (A2BB'O6) solid oxide fuel cell anode material Sr2MgMoO6 is doped with Y so that the high-conductivity double-perovskite-type anode material which is a mixed conductor having a double-perovskite structure is obtained. The preparation method provided by the invention comprises the following steps of pressing B site-doped Sr2Mg1-xYxMoO6 powder (x is in a range of 0.1 to 0.2) into a sample strip under certain pressure, carrying out sintering at a high temperature in an air atmosphere, carrying out reduction under reduction conditions, and carrying out conductivity measuring, wherein compared with a conductivity measured before doping, a conductivity measured after doping is improved 5.8 times (x=0.2). Through the preparation method provided by the invention, the high-conductivity double-perovskite-type anode material which is a porous film-type anode material Sr2Mg1-xYxMoO6 (x is in a range of 0.1 to 0.2) is obtained, and has good combinability and good chemical compatibility with electrolytes of GDC and LSGM, and carbon distribution-resistant and sulfur poisoning-resistant capabilities higher than carbon distribution-resistant and sulfur poisoning-resistant capabilites of the traditional anode material.
Owner:UNIV OF SCI & TECH BEIJING
P5+, Al3+ and B3+ ions co-doped K6Si2O7 potassium fast ion conductor and preparation method thereof
InactiveCN110350250ALower activation energy for migrationLow electronic conductivityFinal product manufactureElectrolytesElectrical conductorActivation energy
The invention relates to a P5+, Al3+ and B3+ ions co-doped K6Si2O7 potassium fast ion conductor and a preparation method thereof. The conductor is characterized in that a stoichiometric formula is K6+x+y-zBxAlyPzSi2-x-y-zO7, wherein x = 0.05~0.10, y = 0.05~0.10, z = 0.02~0.05, and normal temperature potassium ion conductivity exceeds 5*10<-4>S / cm. The conductor is advantaged in that Si4+ ions arepartially replaced with Al3+ and B3+, and interstitial potassium ions are generated in a crystal to reduce potassium ion migration activation energy; electronic conductivity of the fast ion conductoris further reduced by P5+ doping; the size of a migration channel of potassium ions is adjusted by doping of B3+ with a small ionic radius to adapt to rapid migration of the potassium ions; in the preparation process, a surface of the K6Si2O7 particles is modified to form easy-sintering property; the synergistic effect makes potassium ion conductivity of the potassium fast ion conductor exceed 5*10<-4>S / cm, which is closer to the potassium ion conductivity of the liquid electrolyte.
Owner:NINGBO UNIV
P<5+>, A1<3+> and Be<2+> ion collaboratively doped K2MgSi5O12 potassium fast ion conductor and preparation method thereof
InactiveCN110371997ALower activation energy for migrationLow electronic conductivityAluminium silicatesSecondary cellsElectrical conductorActivation energy
The invention discloses a P<5+>, A1<3+> and Be<2+> ion collaboratively doped K2MgSi5O12 potassium fast ion conductor and a preparation method thereof. The P<5+>, A1<3+> and Be<2+> ion collaborativelydoped K2MgSi5O12 potassium fast ion conductor is characterized in that the stoichiometric equation is K<2+2x+y-z>MgBe<x>Al<y>P<z>Si<5-x-y-z>O12, wherein x is within 0.05 to 0.10, y is within 0.05 to 0.10, and z is within 0.02 to 0.05; and the normal-temperature potassium ion conductivity exceeds 5*10<-4> S / cm. A1<3+> and Be<2+> are adopted to partially replace Si<4+> ions, and interstitial potassium ions are generated in crystals to reduce migration activation energy of the potassium ions; the electronic conductivity of the fast ion conductor is further lowered through P<5+> doping; the size of a migration channel of the potassium ions is adjusted through small-ion-radius Be<2+> doping so as to adapt to rapid migration of the potassium ions; and in the preparation process, the surfaces ofK2MgSi5O12 particles are modified, and the easy-to-sinter characteristic is formed. Through the collaborative effect, the normal-temperature potassium ion conductivity of the potassium fast ion conductor exceeds 5*10<-4> S / cm and is closer to the potassium ion conductivity of a liquid electrolyte.
Owner:NINGBO UNIV
A kind of polyolefin-g-polybenzimidazole graft copolymer and its preparation method and application
The invention relates to a polyolefin-g-polybenzimidazole graft copolymer and its preparation method and application. The graft copolymer is the grafting of polyolefin grafted benzimidazole polymer obtained by condensation reaction of the terminal amino group in the benzimidazole polymer and the carboxyl group in the side chain carboxyl-containing olefin polymer. branch copolymers. The proton transport channel is constructed by the phase separation structure of the two chain segments, thereby improving the proton conductivity and achieving a high temperature proton exchange membrane with high proton conductivity under the condition of a low phosphoric acid doping level (ADL<10). The preparation method is simple and easy to operate, and can be applied to fuel cells, liquid flow batteries and the like.
Owner:ZHUHAI COSMX BATTERY CO LTD
Electric field induced crystallization K2.23MgBe0.15P0.07Ti0.03Si4.75O12 potassium fast ion conductor and preparation method thereof
InactiveCN110372360ALower activation energy for migrationIncrease lattice defectsSecondary cellsElectrolytesClose rangePotassium ions
The invention discloses an electric field induced crystallization K2.23MgBe0.15P0.07Ti0.03Si4.75O12 potassium fast ion conductor and a preparation method thereof. The electric field induced crystallization K2.23MgBe0.15P0.07Ti0.03Si4.75O12 potassium fast ion conductor is characterized in that Be<2+> is adopted to partially replace Si<4+> ions, high-concentration interstitial potassium ions are generated in crystals, thus the close-range multiple potassium ions are collaboratively migrated, and migration activation energy of the potassium ions is reduced advantageously; the electronic conductivity of the fast ion conductor is further lowered through P<5+> doping; the size of a migration channel of the potassium ions is adjusted through small-ion-radius Be<2+> doping so as to adapt to rapidmigration of the potassium ions; through Ti<4+> partial doping, distorted lattice structures are formed to increase lattice defects, and thus potassium ion conduction is facilitated; and in the preparation process, the surfaces of K2MgSi5O12 particles are modified, and the easy-to-sinter characteristic is formed. Meanwhile, strong direct-current electric field induced crystallization is introducedto increase the crystallization speed, the crystallization temperature is decreased, and crystallization completeness is improved. Through the collaborative effect, the normal-temperature potassium ion conductivity of the potassium fast ion conductor exceeds 5*10<-4> S / cm and is closer to the potassium ion conductivity of a liquid electrolyte.
Owner:NINGBO UNIV
A liquid phase synthesis k 6.25 be 0.1 al 0.1 p 0.05 ti 0.05 the si 1.7 o 7 Potassium fast ion conductor and preparation method thereof
ActiveCN110526697BLower activation energy for migrationFacilitate conductionSecondary cellsElectrical conductorIonic conductivity
A liquid-phase synthetic K 6.25 be 0.1 al 0.1 P 0.05 Ti 0.05 Si 1.7 o 7 Potassium fast ion conductor and preparation method thereof, is characterized in that: adopt Al 3+ 、Be 2+ Partial replacement of Si 4+ Ions, generate interstitial potassium ions in the crystal and reduce the activation energy of potassium ion migration; through P 5+ Doping further reduces the electronic conductivity of fast ion conductors; through the small ionic radius Be 2+ Doping adjusts the size of the migration channel of potassium ions to adapt to the rapid migration of potassium ions; through Ti 4+ Partial doping forms a distorted lattice structure to increase lattice defects, which is conducive to the conduction of potassium ions; and during the preparation process at K 6 Si 2 o 7 The surface of the particles is modified to form easy sintering properties. These synergistic effects make the normal temperature potassium ion conductivity of this potassium fast ion conductor exceed 5·10 ‑4 S / cm, which is closer to the potassium ion conductivity of the liquid electrolyte.
Owner:NINGBO UNIV
Liquid-phase synthetic K6.4Fe0.05Be0.2Al0.15Ti0.05Si1.6O7 potassium fast ion conductor and preparation method thereof
InactiveCN110330057AReduce grain boundary voidsLower activation energy for migrationSecondary cellsIron compoundsChemistryPotassium ions
The invention discloses a liquid-phase synthetic K6.4Fe0.05Be0.2Al0.15Ti0.05Si1.6O7 potassium fast ion conductor and a preparation method thereof. The liquid-phase synthetic K6.4Fe0.05Be0.2Al0.15Ti0.05Si1.6O7 potassium fast ion conductor is characterized in that Al<3+> and Be<2+> are adopted to partially replace Si<4+> ions, and interstitial potassium ions are produced in a crystal to reduce migration activation energy of potassium ions; the size of a migration channel of potassium ions is adjusted by doping Be<2+> with a small ion radius to adapt rapid migration of potassium ions; a distortedlattice structure is formed by partial doping of Ti<4+>, lattice defects are increased, and potassium ion conduction is facilitated; cation vacancy is formed through partial doping of Fe<3+> to increase the potassium ion migration path; modification is performed on the surface of K6Si2O7 particles in the preparation process, and the easy-to-sinter characteristic is formed. The normal-temperaturepotassium ion conductivity of the potassium fast ion conductor exceeds 5*10<-4> S / cm and is closer to the potassium ion conductivity of a liquid electrolyte under the synergistic effect.
Owner:NINGBO UNIV
K2MgSi5O12 potassium fast ion conductor with Al<3+> and B<3+> synergistically doped and production method of K2MgSi5O12 potassium fast ion conductor with Al<3+> and B<3+> synergistically doped
InactiveCN110371996ALower activation energy for migrationEasy constructionAluminium silicatesSecondary cellsElectrical conductorActivation energy
The invention discloses a K2MgSi5O12 potassium fast ion conductor with Al<3+> and B<3+> synergistically doped and a production method of the K2MgSi5O12 potassium fast ion conductor with the Al<3+> andthe B<3+> synergistically doped. The K2MgSi5O12 potassium fast ion conductor with the Al<3+> and the B<3+> synergistically doped is characterized in that a stoichiometric equation is K(2+x+y)MgBxAlySi(5-x-y)O12, wherein x is 0.05 to 0.15; y is 0.05 to 0.15; and the normal-temperature potassium ion conductivity exceeds 5*10<-4> S / cm. The Al<3+> and the B<3+> are adopted for partially replacing Si<4+> ions, interstitial potassium ions are generated in crystals, and migration activation energy of potassium ions is reduced; by doping the B<3+> with a small ionic radius to adjust the size of a migration channel of the potassium ions so that the migration channel can adapt to fast migration of the potassium ions; and in a production process, modification is conducted on surfaces of K2MgSi5O12 particles, so that the character of easy sintering is formed. Through synergistic effects, the normal-temperature potassium ion conductivity of the potassium fast ion conductor exceeds 5*10<-4> S / cm which is closer to the potassium ion conductivity of a liquid electrolyte.
Owner:NINGBO UNIV
Mg<2+>, Al<3+>, Zr<4+> and S<2-> ion co-doped garnet type solid electrolyte
The invention discloses an Mg<2+>, Al<3+>, Zr<4+> and S<2-> ion co-doped garnet type solid electrolyte Li5La3Nb2O12 which is characterized by comprising the stoichiometric equation: Li[5+x+2y+z]La[3-x]MgxAlyZrzNb[2-y-z]O[12-m]Sm, wherein x is equal to 0.1-0.5, y is equal to 0.1-0.2, z is equal to 0.1-0.2, and m is equal to 0.1-0.3; and the solid electrolyte is formed by uniformly mixing Li2CO3, La2O3, MgO, Al2O3, ZrO2, Nb2O5 and thiourea in the molar ratio of (2.7-3.05):(1.25-1.45):(0.1-0.5):(0.05-0.1):(0.1-0.2):(0.8-0.9):(0.1-0.3), and ball milling, pressing and sintering. According to the invention, the lithium-ion conductivity greater than 10<-4>S / cm can be obtained at room temperature.
Owner:NINGBO UNIV
Electric field induced crystallization A1<3+> and Be<2+> doped K2MgSi5O12 potassium fast ion conductor and preparation method thereof
InactiveCN110372358ALower activation energy for migrationEasy constructionSecondary cellsElectrolytesChemistryElectric field
The invention discloses an electric field induced crystallization A1<3+> and Be<2+> doped K2MgSi5O12 potassium fast ion conductor and a preparation method thereof. The electric field induced crystallization A1<3+> and Be<2+> doped K2MgSi5O12 potassium fast ion conductor is characterized in that the stoichiometric equation is K<2+2x+y>MgBe<x>Al<y>Si<5-x-y>O12, wherein x is within 0.05 to 0.15, andy is within 0.05 to 0.15; and the normal-temperature potassium ion conductivity exceeds 5.10<-4> S / cm. A1<3+> and Be<2+> are adopted to partially replace Si<4+> ions, and interstitial potassium ions are generated in crystals to reduce migration activation energy of the potassium ions; the size of a migration channel of the potassium ions is adjusted through small-ion-radius Be<2+> doping so as toadapt to rapid migration of the potassium ions; and in the preparation process, the surfaces of K2MgSi5O12 particles are modified, and the easy-to-sinter characteristic is formed. Meanwhile, strong direct-current electric field induced crystallization is introduced to increase the crystallization speed, the crystallization temperature is decreased, and crystallization completeness is improved. Through the collaborative effect, the normal-temperature potassium ion conductivity of the potassium fast ion conductor exceeds 5*10<-4> S / cm and is closer to the potassium ion conductivity of a liquid electrolyte.
Owner:NINGBO UNIV
Liquid-phase synthesis multi-ion doped K2MgSi5O12 potassium fast ion conductor and preparation method thereof
InactiveCN110336009ALower activation energy for migrationFacilitate conductionCell electrodesElectrical conductorLattice defects
The invention relates to a liquid-phase synthesis multi-ion doped K2MgSi5O12 potassium fast ion conductor and a preparation method thereof. The liquid-phase synthesis multi-ion doped K2MgSi5O12 potassium fast ion conductor is characterized in that the stoichiometric formula is K2.24MgCa0.05Ba0.02Be0.2P0.02Ti0.02Si4.76O12; and the conductivity of potassium ions at room temperature is higher than 5*10<-4>S / cm. Be2+ partially replace Si4+ ions to produce interstitial potassium ions in a crystal, so as to reduce the activation energy of potassium ion migration. The size of the potassium ion migration channels is adjusted through doping of Be2+ with small ion radius in order to adapt to the rapid migration of potassium ions. A distorted lattice structure is formed through partial doping of Ti4+in order to increase lattice defects and facilitate potassium ion conduction. The electronic conductivity of the fast ionic conductor is further reduced through doping of P5+. Through partial dopingof Ca2+ and Ba2+, cation vacancies are formed to increase potassium ion migration paths. The surface of K2MgSi5O12 particles is modified in the preparation process to make the conductor easy to sinter. The synergistic effect makes the conductivity of potassium ions of the potassium fast ionic conductor exceed 5*10<-4>S / cm at room temperature, which is closer to the conductivity of potassium ions of liquid electrolyte.
Owner:NINGBO UNIV
Liquid phase synthesis K<6.4>Fe<0.05>Cu<0.05>Be<0.2>Al<0.1>B<0.15>Ti<0.02>Si<1.53>O<7> potassium fast ion conductor and preparation method thereof
InactiveCN110364763ALower activation energy for migrationFacilitate conductionSecondary cellsElectrolytesLattice defectsElectrical conductor
The invention relates to a liquid phase synthesis K<6.4>Fe<0.05>Cu<0.05>Be<0.2>Al<0.1>B<0.15>Ti<0.02>Si<1.53>O<7> potassium fast ion conductor and a preparation method thereof. The conductor is characterized in that: Al<3+>, Be<2+>, and B<3+> partially replace Si<4+> ions, and interstitial potassium ions are generated in crystals, so the activation energy of potassium ion migration is reduced; thesize of the migration channels of potassium ions is adjusted by Be<2+> and B<3+> doping with small ionic radius, so the rapid migration of potassium ions is adapted; a distorted crystal lattice structure is formed by partial doping of Ti<4+>, so lattice defects are increased and potassium ion conduction is facilitated; cation vacancies are formed by partial doping of Fe<3+> and Cu<2+>, so the potassium ion migration paths are increased; and in the preparation process, the surface of K6Si2O7 particles is modified, so an easy-sintering property is formed. Through synergistic effects, the normal-temperature potassium ion conductivity of the potassium fast ion conductor exceeds 6*10<-4>S / cm, and is closer to the potassium ion conductivity of the liquid electrolyte.
Owner:NINGBO UNIV
A kind of phosphonated (polyolefin-g-polybenzimidazole) graft copolymer and its preparation method and application
ActiveCN110982081BLower activation energy for migrationPromote migrationFuel cellsPolymer sciencePolyolefin
The invention relates to a phosphonated (polyolefin-g-polybenzimidazole) graft copolymer and its preparation method and application. The graft copolymer of the present invention uses soft polyolefins as the main chain, takes rigid PBI as the branch chain, and further prepares through amino-containing phosphonic acid grafting to obtain both phosphonic acid and soft-hard chains. segmented phosphonated graft copolymers. The polymers with two properties will undergo microscopic phase separation to construct proton transport channels, thereby improving proton conductivity; in addition, the flexible main chain drives the movement of PBI branch chains to reduce the activation energy of proton migration, and promote the migration of phosphoric acid or protons to improve proton conductivity. Rate. The grafted amino-containing phosphonic acid can reduce the doping amount of inorganic phosphoric acid, thereby reducing the loss of phosphoric acid during use, so as to improve the proton conductivity retention rate of the membrane.
Owner:ZHUHAI COSMX BATTERY CO LTD
Fe<2>O<3>/FeF<3-2x>O<x>/Bi<3+> and La<3+> doping ferric fluoride layer structured positive electrode material of lithium battery and preparation method of positive electrode material
ActiveCN105810908AImprove electrochemical performanceImprove conductivityCell electrodesSecondary cellsPhysical chemistryElectron
The invention relates to a Fe<2>O<3> / FeF<3-2x>O<x> / Bi<3+> and La<3+> doping ferric fluoride layer structured positive electrode material of a lithium battery and a preparation method of positive electrode material. After solid-phase synthesis of Bi<3+> and La<3+> doping ferric fluoride, FeF<3-2x>O<x> (x is more than 0 but less than 0.3) and a Fe<2>O<3> layer sequentially wrap Bi<3+> and La<3+> doping ferric fluoride particle according to the characteristic that FeF<3> is easy to be gradually oxidized to Fe<2>O<3> in a relatively high temperature, so that the surface electron conductivity capability of the Bi<3+> and La<3+> doping ferric fluoride is improved, and an adverse effect of an organic electrolyte on the surface of the material particle is resisted; and with the combination of Bi<3+> and La<3+> doping, the comprehensive electrochemical performance of the ferric fluoride is substantially improved.
Owner:NINGBO UNIV
Liquid-phase synthesis K<2.25>MgBe<0.1>Al<0.1>P<0.05>Ti<0.05>Si<4.7>O<12> potassium fast ion conductor and preparation method thereof
ActiveCN110526699ALower activation energy for migrationFacilitate conductionSecondary cellsElectrolytesLattice defectsElectrical conductor
The invention discloses a liquid-phase synthesis K<2.25>MgBe<0.1>Al<0.1>P<0.05>Ti<0.05>Si<4.7>O<12> potassium fast ion conductor and a preparation method thereof. The liquid-phase synthesis K<2.25>MgBe<0.1>Al<0.1>P<0.05>Ti<0.05>Si<4.7>O<12> potassium fast ion conductor is characterized in that electrical conductivity of a potassium ion is greater than 5*10<-4>S / cm at room temperature; Al<3+> and Be<2+> are used for partially replacing Si<4+> ions, and interstitial potassium ions are generated in a crystal to reduce the activation energy of potassium ion migration; the electronic conductivity of the fast ion conductor is further reduced through P<5+> doping; the size of a migration channel of the potassium ion is adjusted through doping of Be<2+> with a small ion radius so as to adapt to the rapid migration of the potassium ions; a distorted lattice structure is formed through Ti<4+> partial doping to increase the lattice defect to facilitate potassium ion conduction; and during the preparation process, the surfaces of K2MgSi5O12 particles are modified to form an easy-to-sinter characteristic. According to the synergistic effects, the electrical conductivity of the potassium ions atthe room temperature of the potassium fast ion conductor is greater than 5*10<-4>S / cm and closer to the electrical conductivity of the potassium ions of liquid electrolyte.
Owner:NINGBO UNIV
A liquid phase synthesis k 2.25 mgbe 0.1 al 0.1 p 0.05 ti 0.05 the si 4.7 o 12 Potassium fast ion conductor and preparation method thereof
ActiveCN110526699BLower activation energy for migrationFacilitate conductionSecondary cellsElectrolytesElectrical conductorFluid phase
A liquid-phase synthetic K 2.25 MgB 0.1 Al 0.1 P 0.05 Ti 0.05 Si 4.7 o 12 Potassium fast ion conductor and preparation method thereof, characterized in that: normal temperature potassium ion conductivity exceeds 5.10 ‑4 S / cm. Using Al 3+ 、Be 2+ Partial replacement of Si 4+ Ions, generate interstitial potassium ions in the crystal and reduce the activation energy of potassium ion migration; through P 5+ Doping further reduces the electronic conductivity of fast ion conductors; through the small ionic radius Be 2+ Doping adjusts the size of the migration channel of potassium ions to adapt to the rapid migration of potassium ions; through Ti 4+ Partial doping forms a distorted lattice structure to increase lattice defects, which is conducive to the conduction of potassium ions; and during the preparation process at K 2 MgSi 5 o 12 The surface of the particles is modified to form easy sintering properties. These synergistic effects make the normal temperature potassium ion conductivity of this potassium fast ion conductor exceed 5·10 ‑4 S / cm, which is closer to the potassium ion conductivity of the liquid electrolyte.
Owner:NINGBO UNIV
K6Si2O7 potassium fast ion conductor co-doped with P5+, Al3+, Be2+ and Zn2+ ions and preparation method thereof
InactiveCN110336008AReduce grain boundary voidsLower activation energy for migrationCell electrodesPotassium ionsRoom temperature
A K6Si2O7 potassium fast ion conductor co-doped with P5+, Al3+, Be2+ and Zn2+ ions and a preparation method thereof are provided. The K6Si2O7 potassium fast ion conductor is characterized in that thestoichiometric formula is K<6+2*x+y-z-2*m>Be<x>Al<y>P<z>Zn<m>Si<2-x-y-z>O<7>, wherein x is equal to 0.1-0.2, y is equal to 0.1-0.2, z is equal to 0.02-0.05, and m is equal to 0.02-0.05; and the conductivity of potassium ions at room temperature is higher than 5*10<-4>S / cm. Al3+ and Be2+ partially replace Si4+ ions to produce interstitial potassium ions in a crystal, so as to reduce the activationenergy of potassium ion migration. The electronic conductivity of the fast ionic conductor is further reduced through doping of P5+. The size of the potassium ion migration channels is adjusted through doping of Be2+ with small ion radius in order to adapt to the rapid migration of potassium ions. Zn2+ partially replaces potassium ions to cause cation vacancies, so as to increase the number of potassium ion migration channels. The surface of K6Si2O7 particles is modified in the preparation process to make the conductor easy to sinter. The synergistic effect makes the conductivity of potassiumions of the potassium fast ionic conductor exceed 5*10<-4>S / cm at room temperature, which is closer to the conductivity of potassium ions of liquid electrolyte.
Owner:NINGBO UNIV
P<5+>, Al<3+>, Be<2+> and Zn<2+> synergistically doped K2MgSi5O12 potassium fast ion conductor and preparation method thereof
InactiveCN110357599ALower activation energy for migrationLow electronic conductivitySecondary cellsElectrolytesElectrical conductorActivation energy
The invention relates to a P<5+>, Al<3+>, Be<2+> and Zn<2+> synergistically doped K2MgSi5O12 potassium fast ion conductor and a preparation method thereof, wherein the stoichiometric formula is K[2+2x+y-z-2m]MgBe[x]Al[y]P[z]Zn[m]Si[5-x-y-z]O12, x is 0.05-0.15, y is 0.05-0.15, z is 0.01-0.03, m is 0.01-0.03, and the conductivity of the potassium ion at a room temperature exceeds 5*10<-4> S / cm. According to the present invention, by partially replacing Si<4+> ions with Al<3+> and Be<2+>, the gap potassium ions are generated in the crystal so as to reduce the potassium ion migration activation energy; through the P<5+> doping, the electron conductivity of the fast ion conductor is further reduced; by doping with the small ionic radius Be<2+>, the size of the potassium ion migration channel isadjusted so as to adapt to the rapid potassium ion migration; by partially replacing the potassium ions with Zn<2+>, the cation vacancy is added so as to increase the potassium ion migration channels; during the preparation, the surface of the K2MgSi5O12 particles is modified so as to form the easy-sintering property; and under the synergistic effects, the room temperature potassium ion conductivity of the potassium fast ion conductor exceeds 5*10<-4> S / cm so as to be close to the potassium ion conductivity of the liquid-state electrolyte.
Owner:NINGBO UNIV
a fe 2 o 3 |fef 3-2x o x |bi 3+ ,la 3+ Doped iron fluoride layer structure lithium battery positive electrode material and preparation method thereof
ActiveCN105810908BImprove electrochemical performanceImprove conductivityCell electrodesSecondary cellsPhysical chemistryIron fluoride
a kind of Fe 2 o 3 |FeF 3‑2x o x |Bi 3+ , La 3+ Doped iron fluoride layer structure lithium battery positive electrode material and preparation method, the method adopts solid-phase synthesis of Bi 3+ , La 3+ After doping iron fluoride, according to FeF 3 It is easy to be gradually oxidized to Fe at higher temperature 2 o 3 The characteristics of Bi 3+ , La 3+ Doped iron fluoride particles coated with FeF in turn 3‑2x o x , 0<x<0.3, and Fe 2 o 3 layer to increase the Bi 3+ , La 3+ The surface electronic conductivity of doped iron fluoride and the resistance to the harmful effects of organic electrolytes on the surface of material particles; combined with Bi 3+ , La 3+ Doping greatly improves the comprehensive electrochemical performance of ferric fluoride.
Owner:NINGBO UNIV
Electric field induced crystallization K<6.25>Be<0.1>Al<0.1>P<0.05>Zr<0.05>Si<1.7>O<7> potassium fast ion conductor and preparation method thereof
InactiveCN110371981ALower activation energy for migrationFacilitate conductionSecondary cellsElectrolytesHigh concentrationElectrical conductor
The invention discloses an electric field induced crystallization K<6.25>Be<0.1>Al<0.1>P<0.05>Zr<0.05>Si<1.7>O<7> potassium fast ion conductor and a preparation method thereof. Al<3+> and Be<2+> are used for partially substituting Si<4+> ions, high-concentration interstitial potassium ions are generated in a crystal, and the reduction of migration activation energy of the potassium ions is facilitated; the electron conductivity of the fast ion conductor is further reduced by doping P<5+>; the size of migration channels of the potassium ions is adjusted by doping Be<2+> with a small ionic radius to adapt to the rapid migration of the potassium ions; Zr<4+> is partially doped to form a distorted lattice structure to increase the lattice imperfectio to facilitate potassium ion conduction; andthe surface of K6Si2O7 particles is modified in the preparation process to form an easy-sintering property. Meanwhile, the introduction of a strong direct current electric field induces crystallization to accelerate the crystallization rate, lower the crystallization temperature and increase the crystal integrity. The synergistic effects enable the normal temperature potassium ion conductivity ofthe potassium fast ion conductor to exceed 5*10<-4> S / cm and to be closer to the potassium ion conductivity of a liquid electrolyte.
Owner:NINGBO UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com