Phosphorylated(polyolefin-g-polybenzimidazole) graft copolymer as well as preparation method and application thereof
A technology of graft copolymer and polybenzimidazole is applied in the field of phosphonated graft copolymer and its preparation, and can solve the problems of loss of phosphoric acid, decrease of proton conductivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0078] The preparation method of the present invention will be further described in detail below in conjunction with specific examples. It should be understood that the following embodiments are only illustrative and explaining the present invention, and should not be construed as limiting the protection scope of the present invention. All technologies implemented based on the foregoing contents of the present invention are covered by the scope of the present invention.
[0079] The experimental methods used in the following examples are conventional methods unless otherwise specified; the reagents and materials used in the following examples, unless otherwise specified, can be obtained from commercial sources.
Embodiment 1
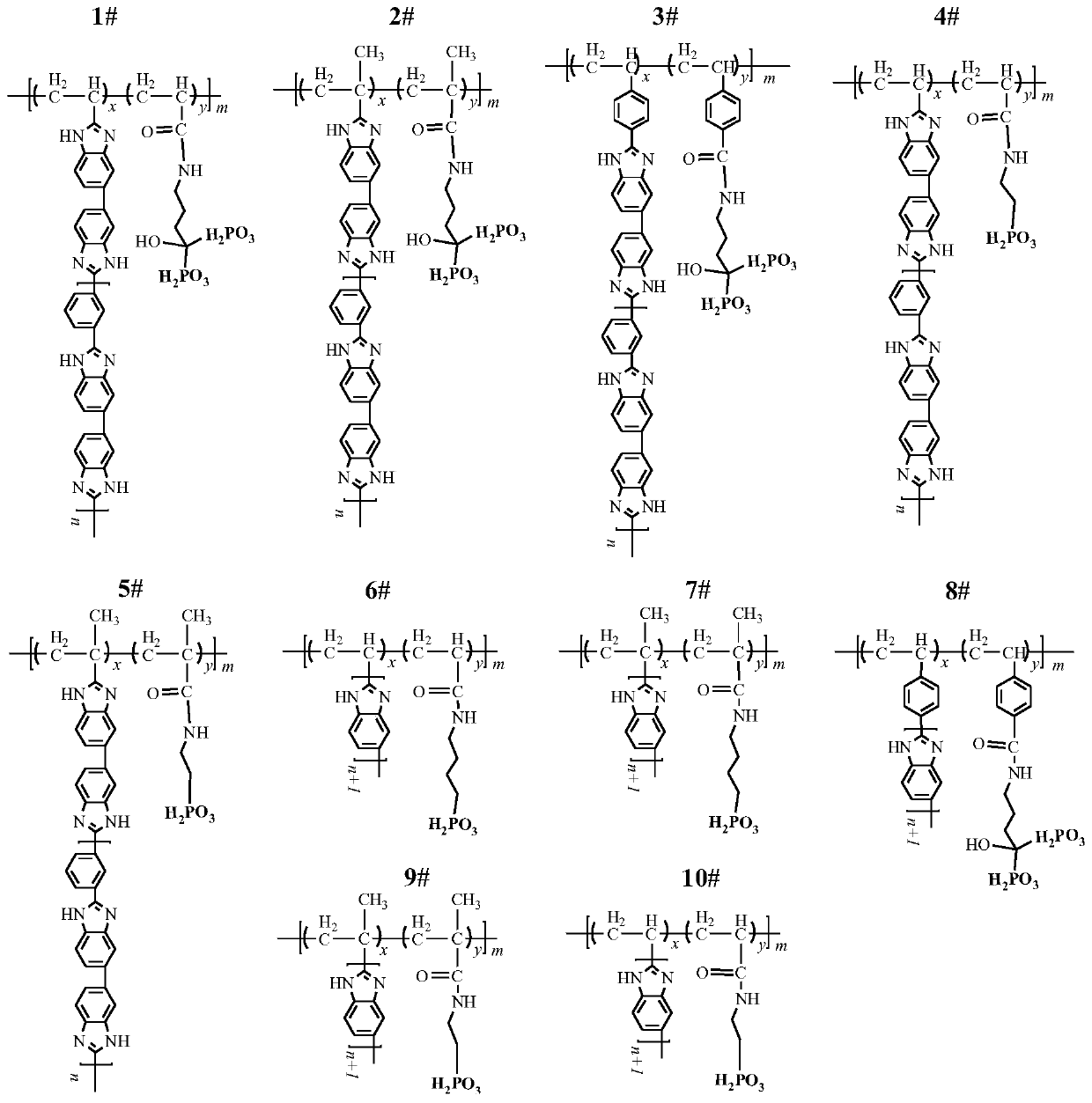
[0103] Phosphonated polyacrylic acid grafted m-polybenzimidazole (P-PAA-g-mPBI, figure 1 Zhong 1#) Preparation of proton exchange membrane
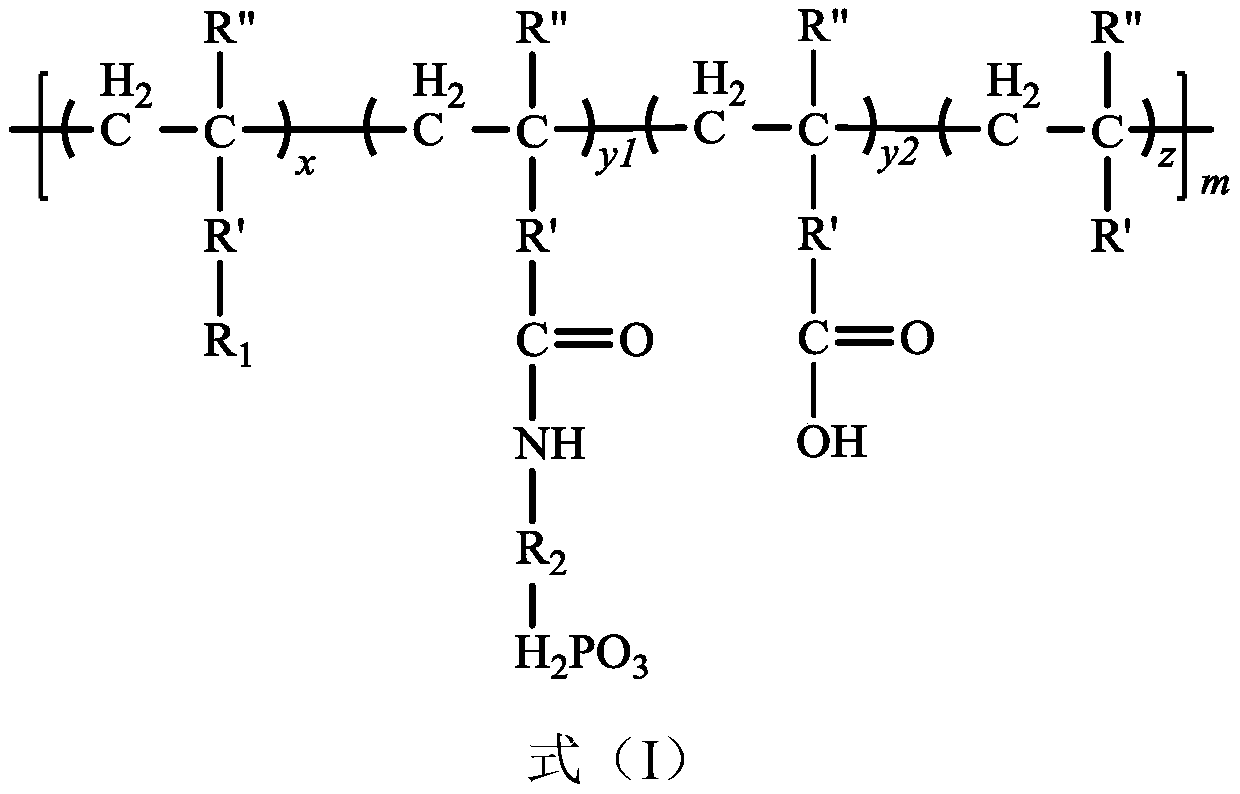
[0104] (1) Dissolve 6.16 g (polymerization degree 100, 0.2 mmol) of mPBI with PBI: carboxyl group = 1:100 (molar ratio) and polyacrylic acid 1.44 g (20 mmol carboxyl group) in 100 mL of DMF, and react at 160° C. for 15 h.
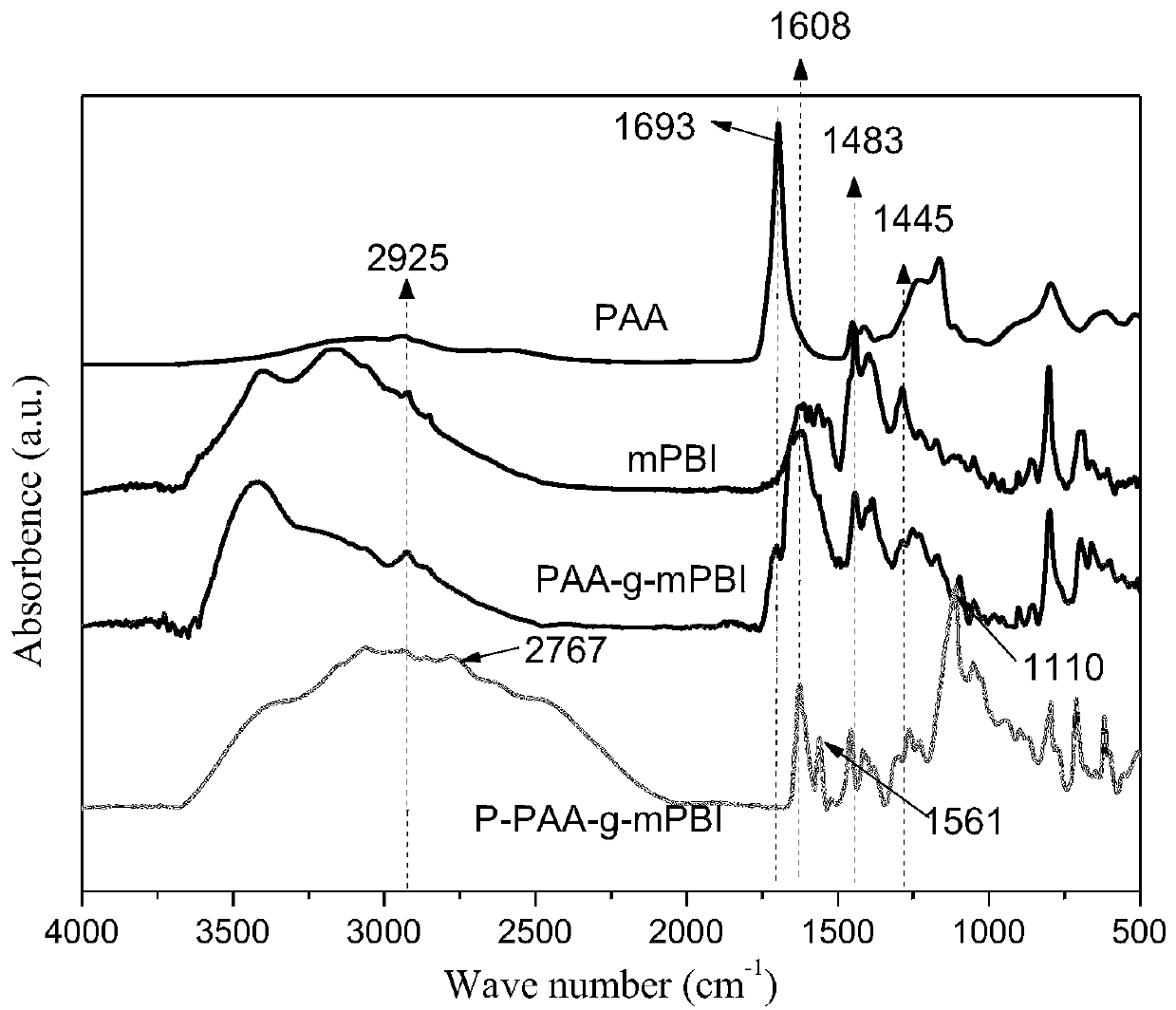
[0105] (2) After the reaction is completed, add 5.916 g of amino-containing alendronic acid (1.2 times the molar amount of carboxyl groups in the remaining PAA) to the above solution, reflux and stir the reaction at 160°C for 10 hours, and then rotate the solvent after the reaction is completed Control the total solid content to 10%, then pour the solution onto the glass plate and coat it with a 400μm doctor blade, and then volatilize the solvent at 60~120℃. After the solvent is completely volatilized, perform a 400μm doctor blade coating and dry to get 65μm The P-PAA-g-mPBI film.
[0106] (3) The P-PAA-g-mPBI membrane was i...
Embodiment 2
[0110] Phosphonated polymethacrylic acid grafted m-polybenzimidazole (P-PMAA-g-mPBI, figure 1 2#) Preparation of proton exchange membrane
[0111] (1) Dissolve 9.24g (polymerization degree 50, 0.6mmol) of mPBI with a molar ratio of PBI: carboxyl group=1:33.33 (molar ratio) and PMAA 1.74g (20mmol carboxyl group) in 100mL DMF, and react at 160℃ 15h.
[0112] (2) The same as Example 1, except that the amino group-containing phosphonic acid is 5.797 g of alendronic acid (1.2 times the molar amount of carboxyl groups in the remaining PMAA).
[0113] (3) Same as Example 1.
[0114] After testing, the ADL of the high temperature proton exchange membrane is 9.09, the proton conductivity at 180°C is 0.0888S / cm, the proton conductivity after 10 deionized water immersion is 0.0703S / cm, and the conductivity retention rate is 79.3 %, compared with the mPBI conductivity retention rate (69.7%) in Comparative Example 1, the improvement ratio is 13.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Proton conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Proton conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com