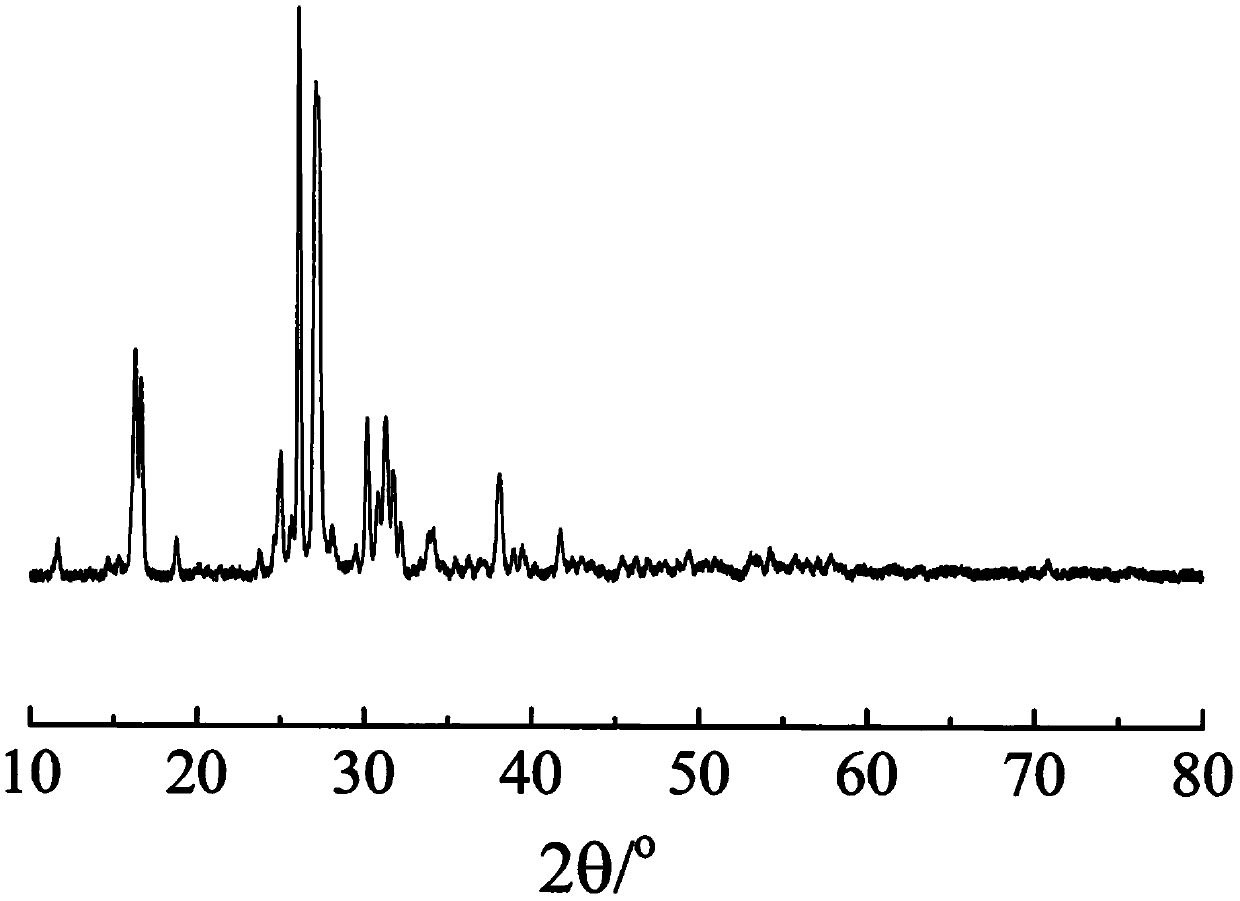
Liquid-phase synthesis multi-ion doped K2MgSi5O12 potassium fast ion conductor and preparation method thereof
A technology of ion doping and liquid phase synthesis, applied in electrical components, battery electrodes, circuits, etc., to reduce electronic conductivity and reduce grain boundary voids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Embodiment 1: the solid KNO 3 : NH 4 h 2 PO 4 : Ca(NO 3 ) 2 2H 2 O:Ba(NO 3 ) 2 : Mg(NO 3 ) 2 ·6H 2 O according to K 2.24 MgCa 0.05 Ba 0.02 be 0.2 P 0.02 Ti 0.02 Si 4.76 o 12 The ratio of the stoichiometric molar ratio of the corresponding elements in the mixture is uniformly mixed, while vigorously stirring, add deionized water until all solid substances are dissolved, record the mass of the added deionized water, and then continue to add the recorded deionized water 1.1 times the quality of deionized water and stir evenly, then continue to stir and add 35wt% beryllium nitrate aqueous solution until the amount of beryllium in the solution system meets K 2.24 MgCa 0.05 Ba 0.02 be 0.2 P 0.02 Ti 0.02 Si 4.76 o 12 The stoichiometric ratio, and the amount of tartaric acid added is 1.6 times the total amount of all metal ions and fully stirred until completely dissolved; record this solution as solution A; will meet K 2.24 MgCa 0.05 Ba 0.02 be 0.2...
Embodiment 2
[0012] Embodiment 2: the solid KNO 3 : NH 4 h 2 PO 4 : Ca(NO 3 ) 2 2H 2 O:Ba(NO 3 ) 2 : Mg(NO 3 ) 2 ·6H 2 O according to K 2.24 MgCa 0.05 Ba 0.02 be 0.2 P 0.02 Ti 0.02 Si 4.76 o 12 The ratio of the stoichiometric molar ratio of the corresponding elements in the mixture is uniformly mixed, while vigorously stirring, add deionized water until all solid substances are dissolved, record the mass of the added deionized water, and then continue to add the recorded deionized water 1.5 times the quality of deionized water and stir evenly, then continue to stir and add 35wt% beryllium nitrate aqueous solution until the amount of beryllium in the solution system meets K 2.24 MgCa 0.05 Ba 0.02 be 0.2 P 0.02 Ti 0.02 Si 4.76 o 12 The stoichiometric ratio, and the amount of tartaric acid added is 2.3 times the total amount of all metal ions and fully stirred until completely dissolved; record this solution as solution A; will meet K 2.24 MgCa 0.05 Ba 0.02 be 0.2...
Embodiment 3
[0013] Embodiment 3: the solid KNO 3 : NH 4 h 2 PO 4 : Ca(NO 3 ) 2 2H 2 O:Ba(NO 3 ) 2 : Mg(NO 3 ) 2 ·6H 2 O according to K 2.24 MgCa 0.05 Ba 0.02 be 0.2 P 0.02 Ti 0.02 Si 4.76 o 12The ratio of the stoichiometric molar ratio of the corresponding elements in the mixture is uniformly mixed, while vigorously stirring, add deionized water until all solid substances are dissolved, record the mass of the added deionized water, and then continue to add the recorded deionized water 1.3 times the quality of deionized water and stir evenly, then continue to stir and add 35wt% beryllium nitrate aqueous solution until the amount of beryllium in the solution system meets K 2.24 MgCa 0.05 Ba 0.02 be 0.2 P 0.02 Ti 0.02 Si 4.76 o 12 The stoichiometric ratio, and the amount of tartaric acid added is 2.0 times the total amount of all metal ions and fully stirred until completely dissolved; record this solution as solution A; will meet K 2.24 MgCa 0.05 Ba 0.02 be 0.2 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com