A kind of phosphonated (polyolefin-g-polybenzimidazole) graft copolymer and its preparation method and application
A technology of graft copolymer and polybenzimidazole, which is applied in the field of phosphonated graft copolymer and its preparation, can solve the problems of decreased proton conductivity and loss of phosphoric acid, and can improve proton conductivity, reduce loss, and increase proton Effect of conductivity retention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0078] The preparation method of the present invention will be further described in detail in conjunction with specific examples below. It should be understood that the following examples are only for illustrating and explaining the present invention, and should not be construed as limiting the protection scope of the present invention. All technologies realized based on the above contents of the present invention are covered within the scope of protection intended by the present invention.
[0079] The experimental methods used in the following examples are conventional methods unless otherwise specified; the reagents and materials used in the following examples can be obtained from commercial sources unless otherwise specified.
Embodiment 1
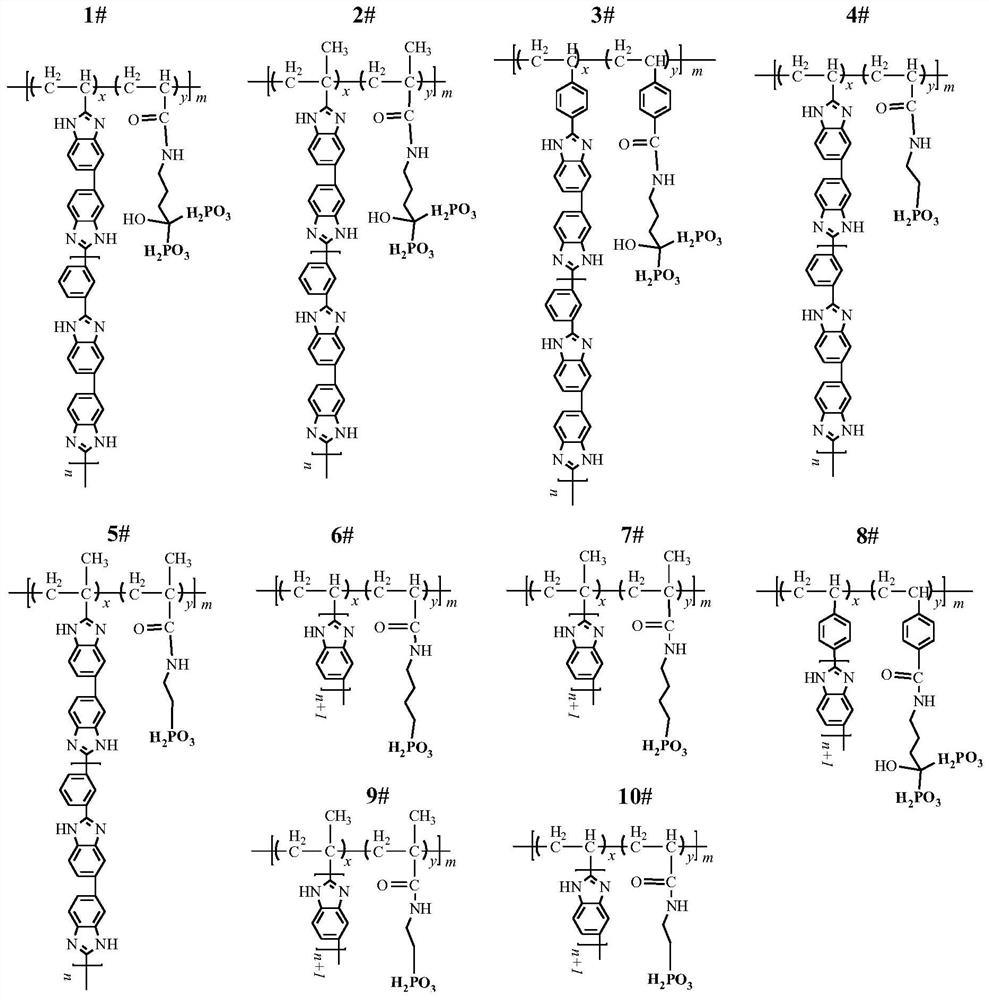
[0103] Phosphonated polyacrylic acid grafted with m-polybenzimidazole (P-PAA-g-mPBI, figure 1 Middle 1#) Preparation of proton exchange membrane
[0104] (1) Dissolve 6.16g of mPBI (polymerization degree 100, 0.2mmol) and polyacrylic acid 1.44g (20mmol carboxyl group) of PBI: carboxyl = 1:100 (molar ratio) in 100mL of DMF and react at 160°C for 15h.
[0105] (2) After the reaction, add 5.916 g of amino-containing phosphonic acid alendronic acid (1.2 times the molar weight of the carboxyl groups in the remaining PAA) to the above solution, and reflux and stir the reaction at 160°C for 10 hours. After the reaction, the solvent is rotary evaporated Control the total solid content to 10%, then pour the solution on a glass plate and apply it with a 400μm scraper, then volatilize the solvent at 60-120°C, and after the solvent is completely volatilized, apply a 400μm scraper and dry to get 65μm P-PAA-g-mPBI membrane.
[0106] (3) The above P-PAA-g-mPBI membrane was soaked in 85% ph...
Embodiment 2
[0110] Phosphonated polymethacrylic acid grafted m-polybenzimidazole (P-PMAA-g-mPBI, figure 1 Middle 2#) preparation of proton exchange membrane
[0111] (1) Dissolve mPBI 9.24g (polymerization degree 50, 0.6mmol) and PMAA 1.74g (20mmol carboxyl) with a molar ratio of PBI: carboxyl = 1:33.33 (molar ratio) in 100mL of DMF and react at 160°C 15h.
[0112] (2) Same as Example 1, except that the amino-containing phosphonic acid is 5.797 g of alendronic acid (1.2 times the carboxyl molar weight in the remaining PMAA).
[0113] (3) Same as Example 1.
[0114] After testing, the ADL of the high-temperature proton exchange membrane is 9.09, the proton conductivity at 180°C is 0.0888S / cm, the proton conductivity after 10 deionized water immersions is 0.0703S / cm, and the conductivity retention rate is 79.3 %, compared to the mPBI conductivity retention rate (69.7%) in Comparative Example 1, the improvement ratio was 13.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com