Electric field induced crystallization A1<3+> and Be<2+> doped K2MgSi5O12 potassium fast ion conductor and preparation method thereof
An electric field induction, ion conductor technology, applied in the direction of circuits, electrolytes, electrical components, etc., to reduce the activation energy of migration, speed up the speed, and reduce the effect of grain boundary voids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
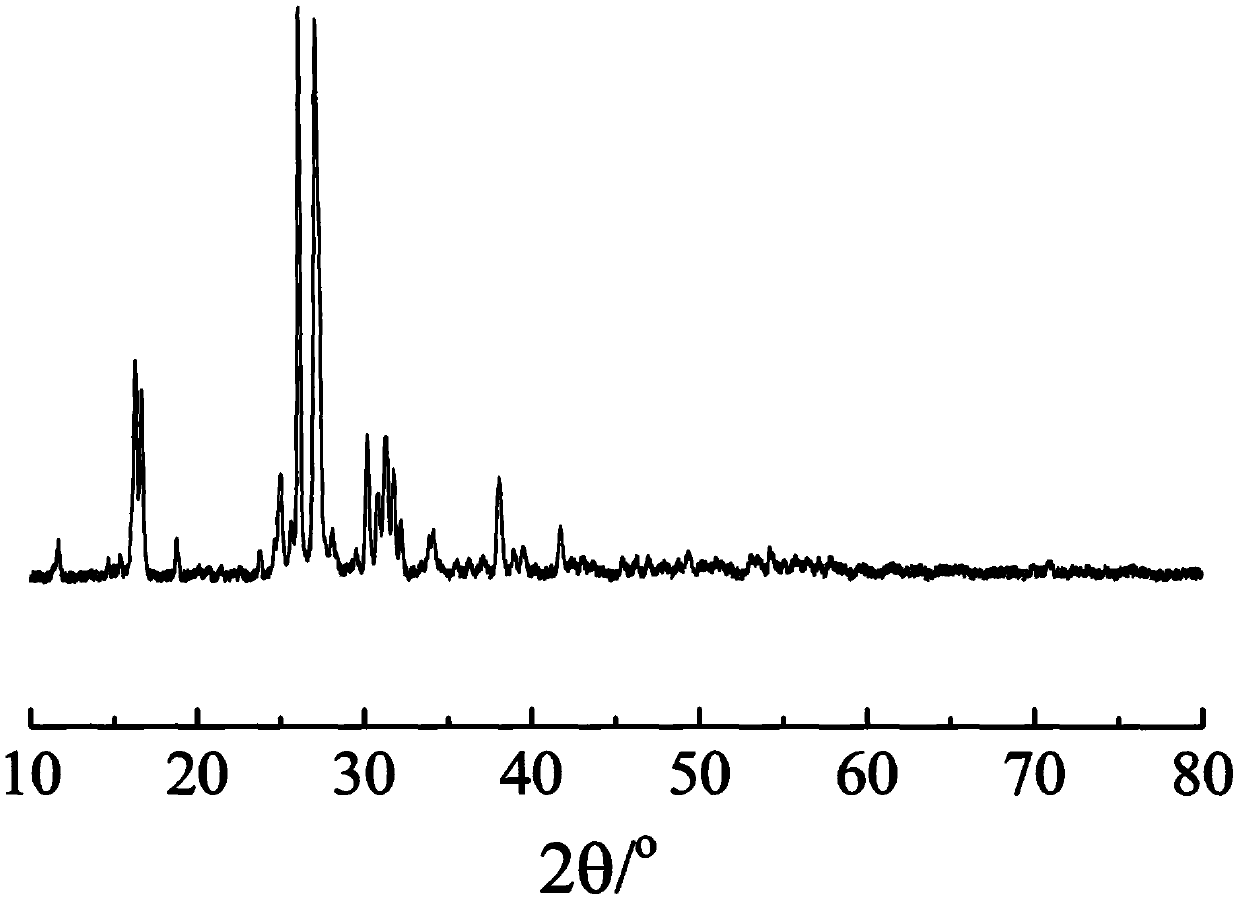
Image
Examples
Embodiment 1
[0011] Embodiment 1: the solid K 2 CO 3 :MgO:Al 2 o 3 : SiO 2 : BeO according to K 2.15 MgB 0.05 al 0.05 Si 4.89 o 12 The ratio of the stoichiometric molar ratio is evenly mixed, adding quality is the dehydrated alcohol of mixture quality 3%, in ball mill with the rotating speed ball mill of 120 rev / mins 10 hours, after ball mill finishes, in 60 ℃ of vacuum ovens (gas pressure in oven is at 5 Pa) for 3 hours, take it out and re-grind in an agate mortar for 12 minutes, the ground powder is heated to 550 ° C at a rate of 7 ° C / min in the air atmosphere and then cooled with the furnace for 3 hours; The powder was ground again in an agate mortar for 10 minutes, and the ground powder was heated up to 1250 °C at a rate of 5 °C / min in a platinum crucible in an air atmosphere and kept for 25 hours, then taken out of the furnace and cooled rapidly; the cooled The material was pulverized and ground for 30 minutes in the mill, soaked in 0.1 M sodium hydroxide solution for 5 mi...
Embodiment 2
[0012] Embodiment 2: the solid K 2 CO 3 :MgO:Al 2 o 3 : SiO 2 : BeO according to K 2.45 MgB 0.15 al 0.15 Si 4.7 o 12 The ratio of the stoichiometric molar ratio is evenly mixed, adding quality is the dehydrated alcohol of mixture quality 9%, in ball mill with the rotating speed ball mill of 400 rev / mins 40 hours, after ball mill finishes, in 110 ℃ of vacuum ovens (gas pressure in oven is at 20 Pa) for 10 hours, take it out and re-grind in an agate mortar for 30 minutes, heat up the ground powder at a rate of 30 °C / min to 600 °C for 10 hours in an air atmosphere, and then cool it with the furnace; The powder was ground again in an agate mortar for 30 minutes, and the ground powder was heated to 1330°C in a platinum crucible in an air atmosphere at a rate of 13°C / min and kept at 1330°C for 45 hours, then taken out of the furnace and cooled rapidly; the cooled The material was pulverized and ground for 50 minutes in the mill, soaked in 0.3M sodium hydroxide solution for ...
Embodiment 3
[0013] Embodiment 3: the solid K 2 CO 3 :MgO:Al 2 o 3 : SiO 2 : BeO according to K 2.3 MgB 0.1 al 0.1 Si 4.8 o 12 The ratio of the stoichiometric molar ratio is uniformly mixed, adding quality is the dehydrated alcohol of mixture quality 6%, in ball mill with the rotating speed ball mill of 300 rev / mins 30 hours, after ball mill finishes, in 100 ℃ of vacuum ovens (gas pressure in oven is at 15Pa) for 6 hours, take it out and re-grind in an agate mortar for 20 minutes, the ground powder is heated to 600°C at a rate of 20°C / min in an air atmosphere and then cooled with the furnace for 7 hours; The powder was ground again in an agate mortar for 20 minutes, and the ground powder was heated up to 1300°C in a platinum crucible in an air atmosphere at a rate of 10°C / min and kept at 1300°C for 35 hours, then taken out of the furnace and cooled rapidly; the cooled The material was pulverized and ground for 40 minutes in the mill, soaked in 0.2M sodium hydroxide solution for 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com