Treatment of nasal and sinus disorders
A sinus, disease technology, applied in the treatment of nasal and sinus disorders, can solve problems such as unpredictability, leakage of gauze delivery, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0089] Example 1: Topical Nasal Administration
[0090] A 30-year-old woman presented with allergic rhinitis manifested by postnasal drip, congestion, and sneezing. She also had allergic asthma manifested by wheezing and coughing.
[0091] 200 units of diffusible BoNT in 2cc of gel carrier were placed on the external nares for 30 minutes and then wiped off. The BoNT diffuses across the skin to reach the inner nasal mucosa. After 1 week, the patient noted a decrease in sneezing and nasal itching. Pulmonary function tests showed decreased bronchial reactivity and coughing.
[0092] Alternatively, approximately 1 cc of the gel carrier can be placed on the skin of the nasal vestibule.
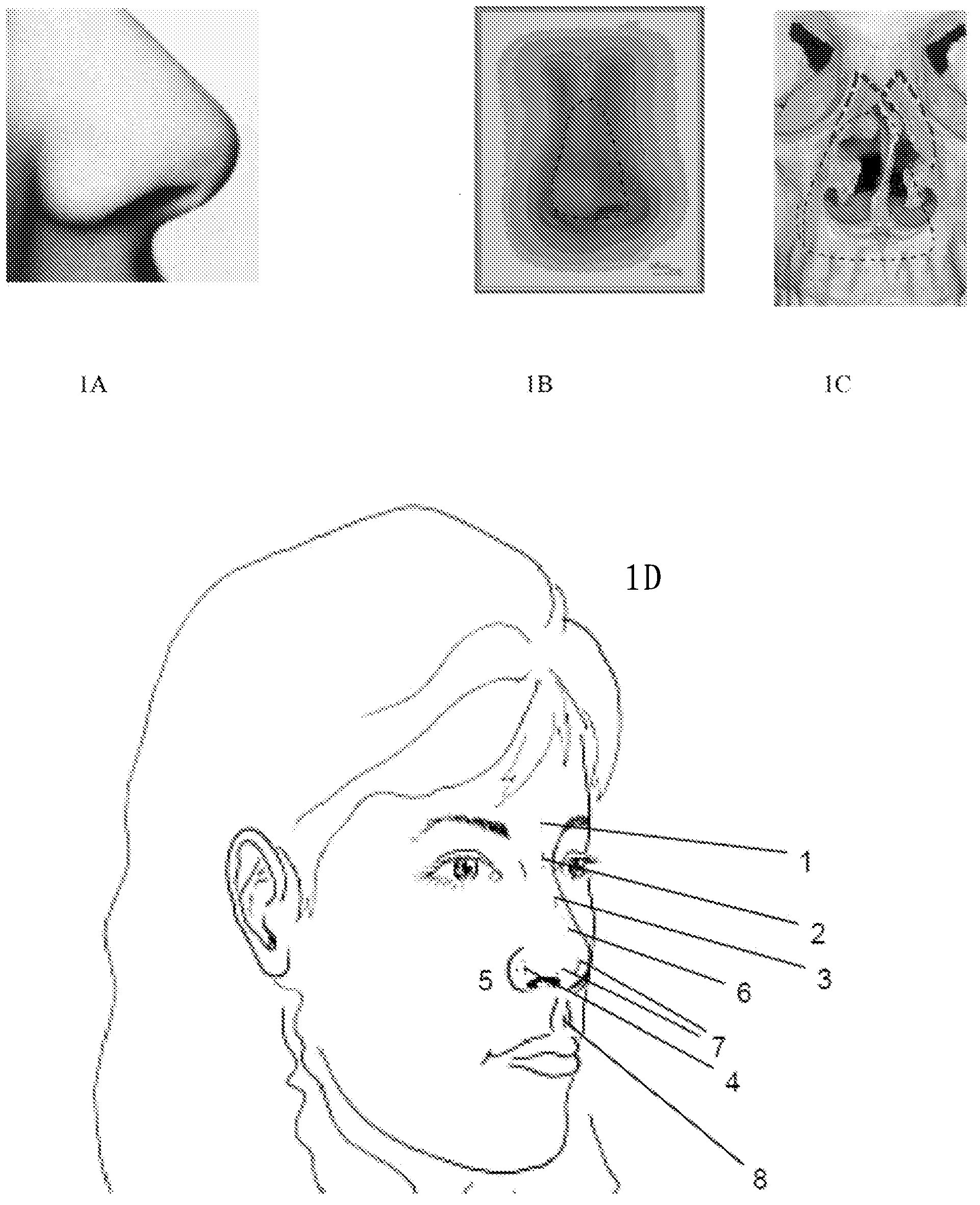
[0093] Dermal areas where BoNT can be administered are shown in Figure 8 middle. The preferred mucous membrane for allergic rhinitis is the most anterior portion of the nasal cavity.
example 2
[0094] Example 2: Needle Injection
[0095] Needle injections can be shown at Figure 8 Any reticular lines in the area are carried across the outer nasal skin. Once through the skin, BoNT can be injected into soft tissue and allowed to diffuse to the nasal mucosa. However, if the needle is advanced to a mucosal target, the need for diffusion and more efficient delivery is lower. In some embodiments, the needle can be advanced directly to the nasal mucosa. In other embodiments, the needle can then pass through the nasal mucosa and thence into the nasal cavity where it can be redirected to inject multiple areas.
[0096] A 20-year-old male complained of seasonal sneezing and congestion and was diagnosed with allergic rhinitis. His physician injected 20 units of BoNT mixed with .5 cc of saline into the anterior tip of each inferior turbinate. Specifically, the physician feels the patient's face to find the bony borders of the nostrils. A 1 cc syringe with a 1"32 gauge ne...
example 3
[0097] Example 3: Transdermal Pressure Injection
[0098] Pressure syringes propel medication by brief pulses of highly pressurized gas. Unexpectedly, large molecules such as BoNT can be propelled across skin, subcutaneous tissue, bone, fat, and mucous membranes. The injection can be in a straight line into the tissue or at an angle. Injections can be held in tissue or passed into the nasal cavity. Injection depth can vary from .1mm to 20mm. Injection volumes can vary from .01ml to 10ml. Pressure ranges, particle sizes and velocities are taught in the references cited above.
[0099]The nozzle of the pressure syringe can be administered at the external nostril at an angle facing the endonasal mucosa; at the piriform foramen at an angle facing the endonasal mucosa; above these bones by injection through the nasal bones; by using Injections through the skin and into these structures are administered over the lacrimal sac or duct; under the lip against the gums with an ang...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com