Antagonism of human formyl peptide receptor for treatment of disease
A methyl and alkyl technology, applied in the field of antagonism of human formyl peptide receptors for the treatment of diseases, can solve problems such as toxicity hindering long-term use, side effects hindering widespread or universal use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
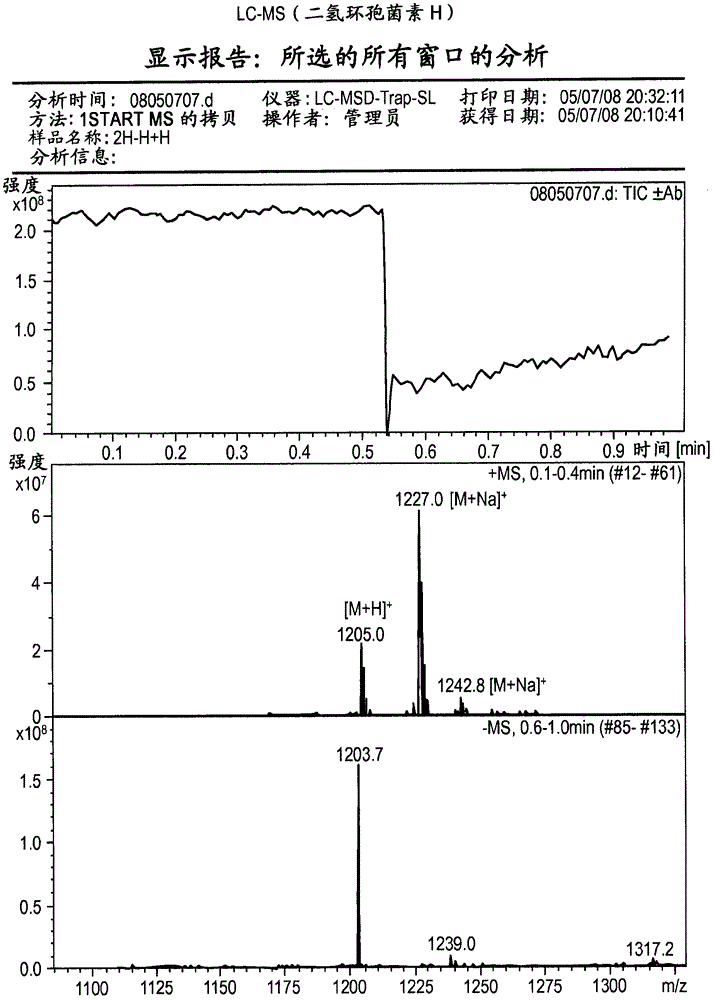
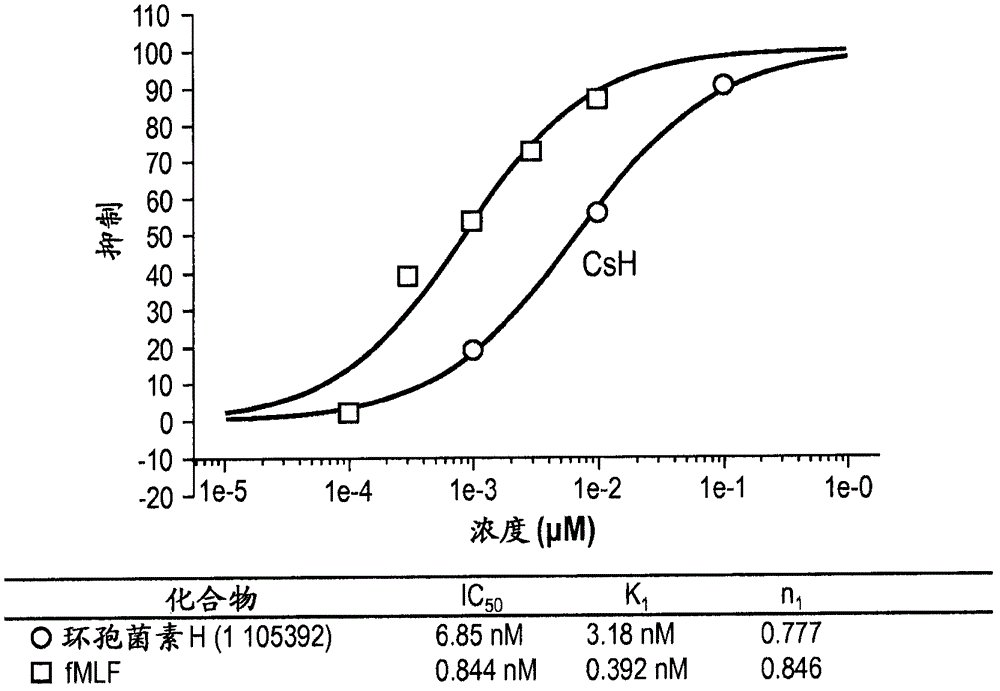
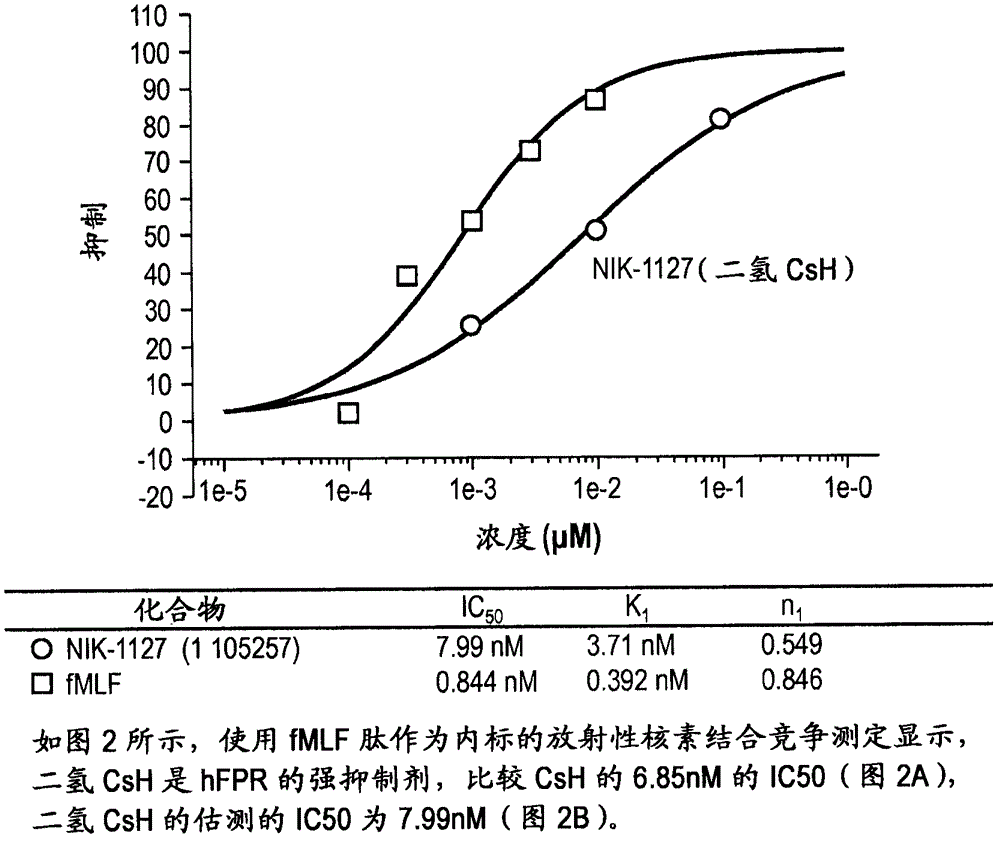
[0044] Cyclosporin H (CsH) is known to be a specific antagonist of formyl peptide receptors (Wenzel-Seifert et al., 1991; Wenzel-Seifert and Seifert, 1993). Provided herein are therapeutic uses of FPR antagonists for the treatment of eosinophil-associated and neutrophil-associated diseases and disorders, including those listed above. In preferred embodiments, such antagonists include CsH and derivatives thereof that act as potent and specific FPR antagonists (eg, dihydroCsH and compounds of formula I). The cyclosporins of the present invention are administered at doses that produce a reduction in circulating eosinophils or neutrophils or a reduction in eosinophils or neutrophils in contrast to eosinophils or neutrophils. A decrease in disease-associated sites of increased granulocyte function or local cell numbers, or a decrease in the activation of eosinophils or neutrophils at such sites.
[0045] Compounds of the invention
[0046] In one aspect, the present invention p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com