Tricyclic indole derivatives as PBR ligands
A precursor compound and compound technology, applied in the field of positron-emission tomography X-ray imaging, preparation of the precursor compound and the PET tracer, radiopharmaceutical composition, and synthesis of the precursor compound, It can solve the problems of low non-specific binding specificity, unknown role of metabolites, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
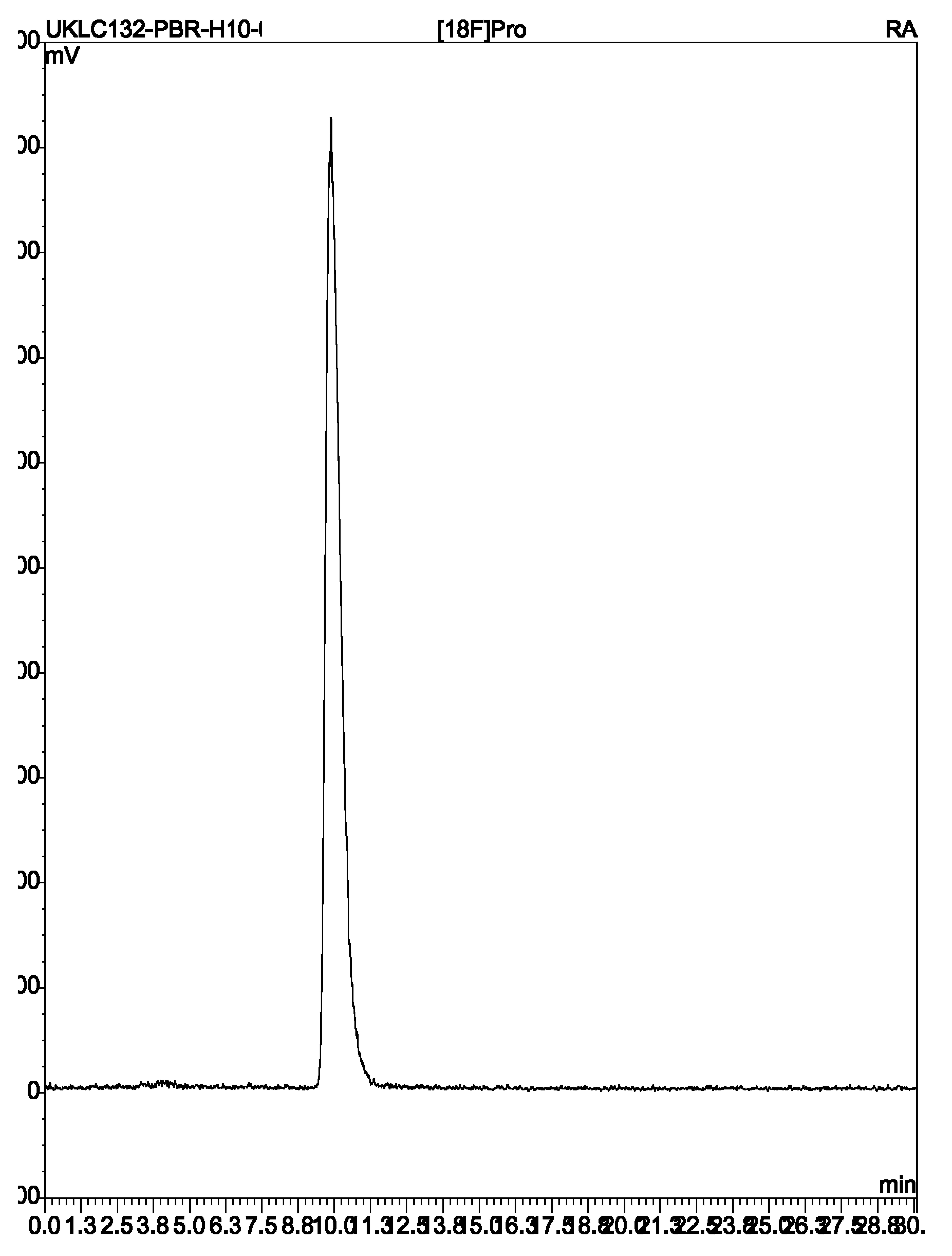
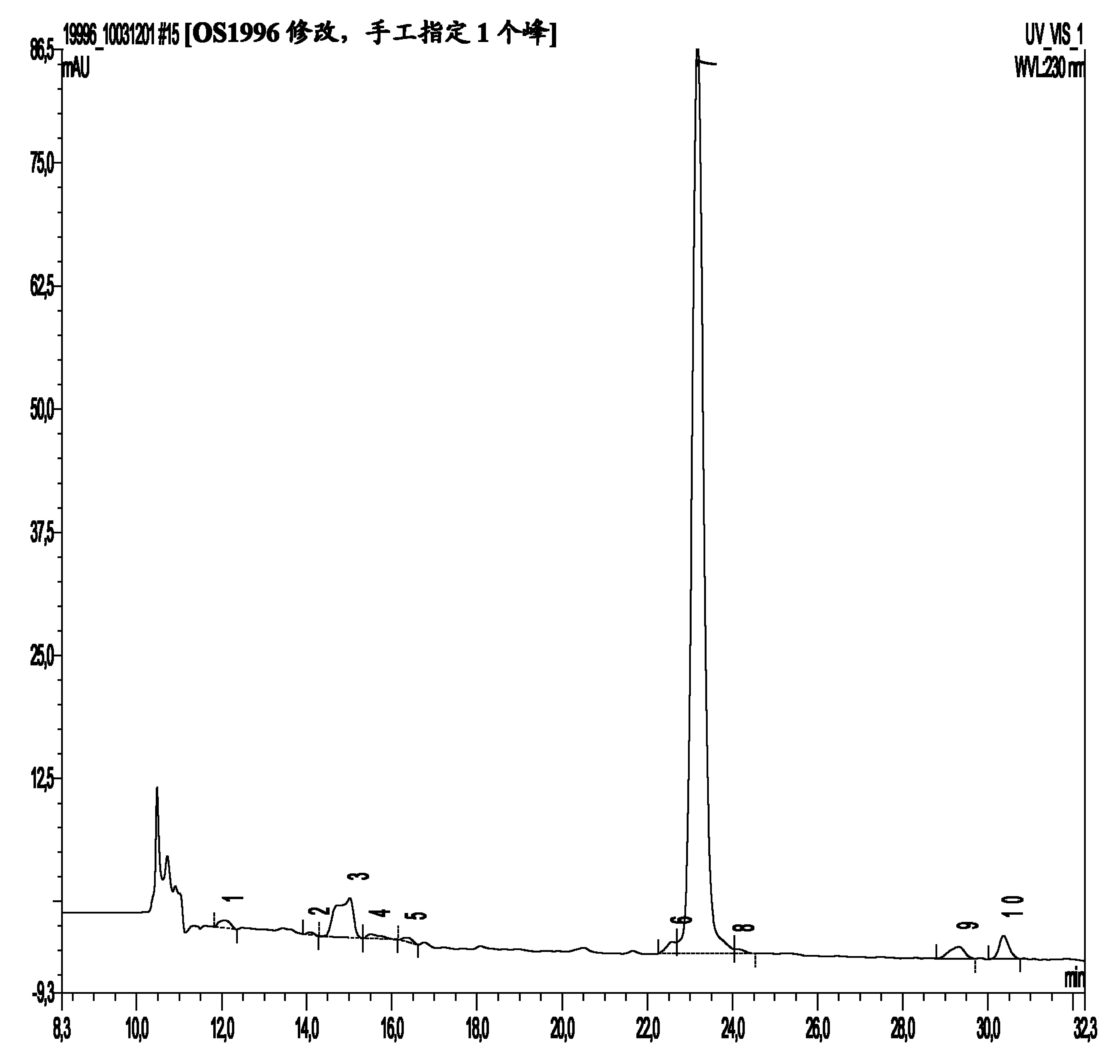
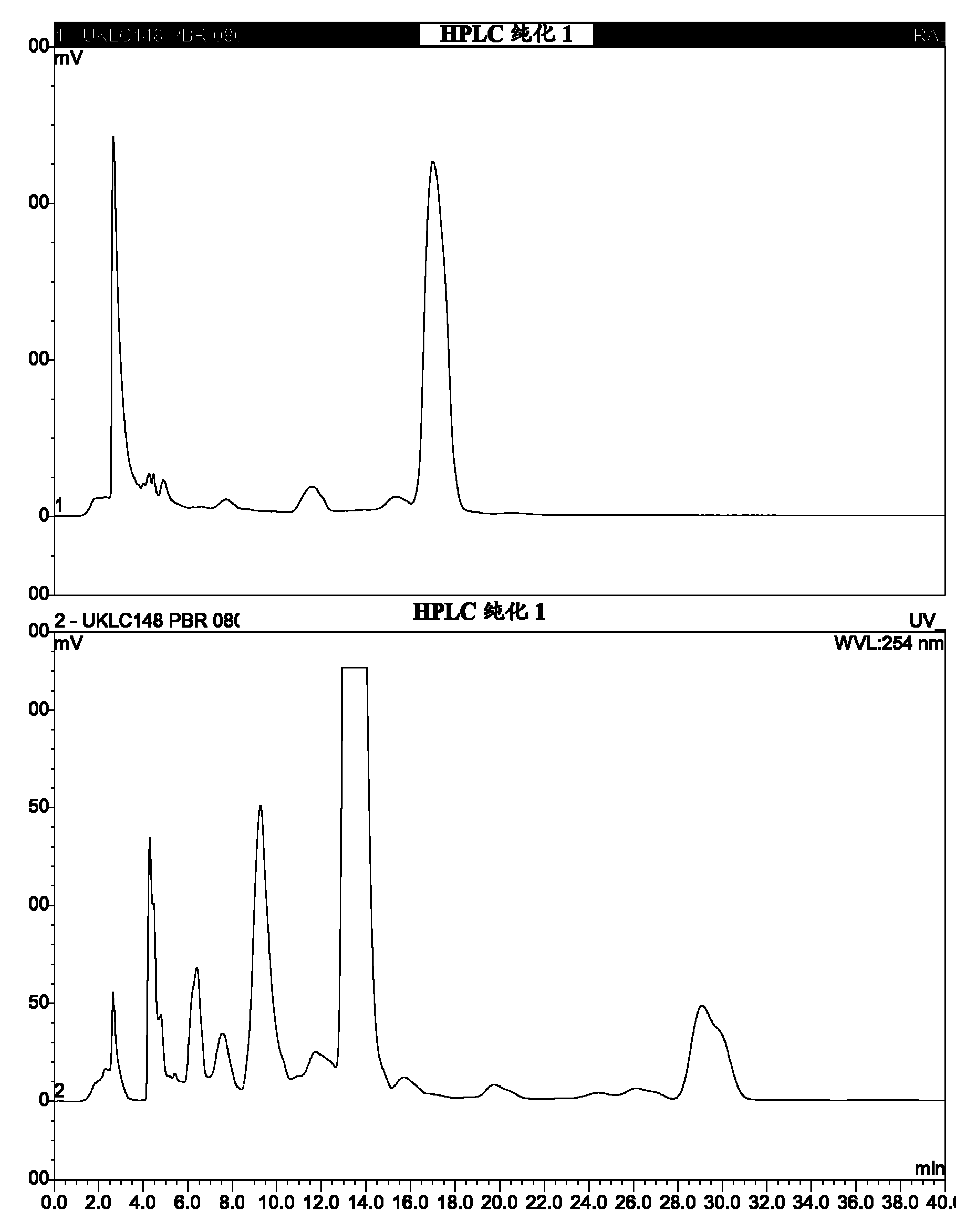
Image
Examples
preparation example Construction
[0029] Preparation of precursor compounds
[0030] The precursor compounds of the invention may be obtained by a number of different routes, each forming a separate aspect of the invention.
[0031] Accordingly, the present invention provides a first process for the preparation of a precursor compound of formula I as defined herein, wherein said process comprises:
[0032] (i) providing a racemic mixture of said precursor compound of formula I and a compound of formula II as defined herein:
[0033] (II);
[0034] where R 2 As above for R 1 defined by, and R 1 and R 2 same;
[0035] (ii) separating said precursor compound of formula I from said compound of formula II.
[0036] The step of "separating" said precursor compound of formula I from said compound of formula II is carried out by enantiomeric separation techniques. Suitable enantiomeric separation techniques include high performance liquid chromatography (HPLC), supercritical fluid chromatography (SFC), simul...
Embodiment 1
[0113] Example 1 describes the synthesis of a racemic mixture comprising a precursor compound of Formula I and an enantiomer of Formula II.
Embodiment 2
[0114] Example 2 describes the synthesis of a non-radioactive racemic mixture comprising a non-radioactive analog of a PET tracer of the present invention as well as its alternative enantiomers.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com