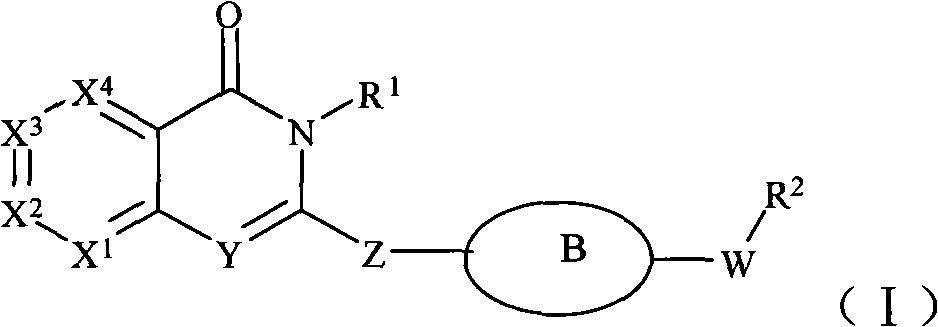
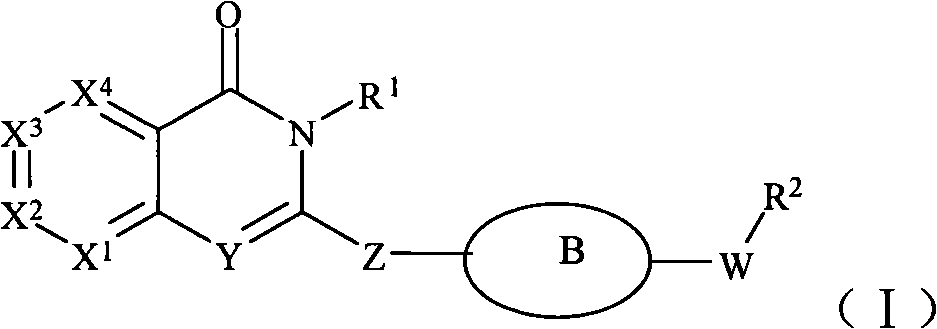
Phenylquinazoline PI3Kdelta inhibitors
A technology of alkyl and amino groups, applied in anti-inflammatory agents, non-central analgesics, active ingredients of heterocyclic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
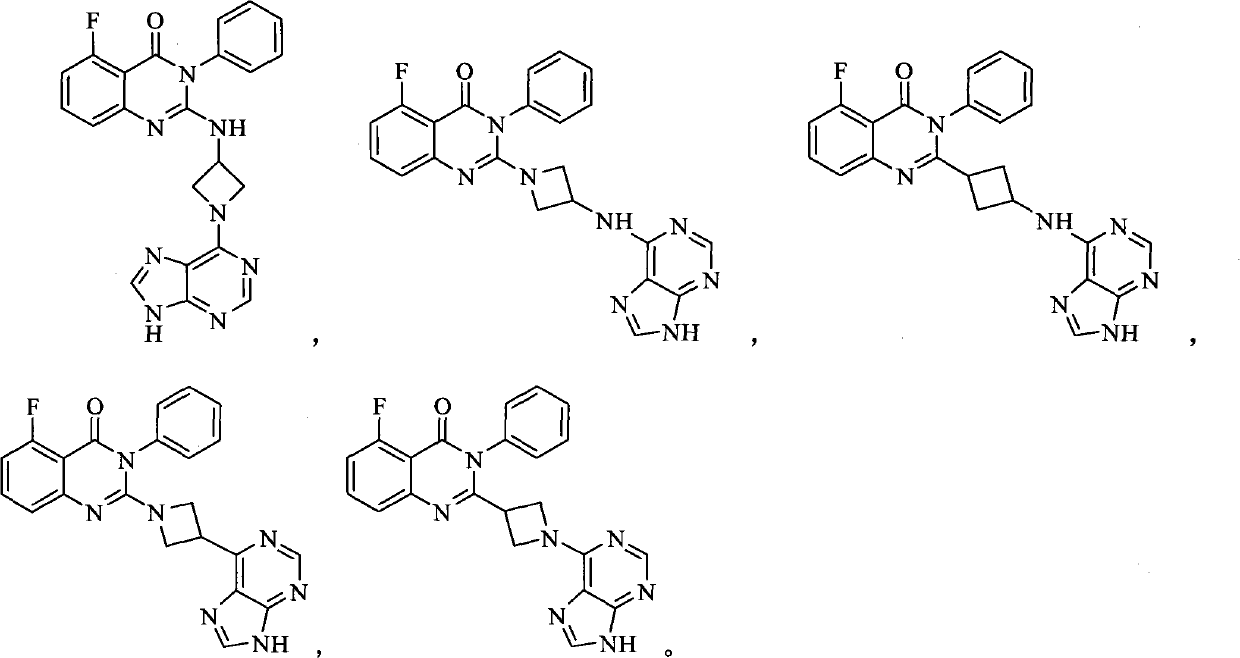
[0140] Example 1 Preparation of 2-(1-(9H-purin-6-yl)azetidinyl-3-ylamino)-5-fluoro-3-phenylquinazolin-4(3H)-one (compound 1)
[0141]
[0142] (1) Preparation of 2-fluoro-6-nitro-N-phenylbenzamide
[0143]
[0144] Add 2-fluoro-6-nitrobenzoic acid (5.5g, 30mmol) into 40mL of dichloromethane, add DMF 1mL dropwise, add oxalyl chloride (6.7g, 50mmol) dropwise within 30min under ice-cooling, and stir at room temperature for 2h , the reaction solution was spin-dried, dissolved in dichloromethane, slowly added dropwise to the dichloromethane solution of aniline (3.1g, 30mmol) and triethylamine (5.05g, 50mmol) under ice bath, controlled temperature at 10°C, stirred for 2h , add water 300mL, precipitate out, filter and dry to obtain product 7.02g, yield: 90%.
[0145] (2) Preparation of 2-amino-6-fluoro-N-phenylbenzamide
[0146]
[0147] Add 2-fluoro-6-nitro-N-phenylbenzamide (26g, 1000mmol), zinc powder (195g, 300mmol), ammonium chloride (160g, 300mmol) into 100mL tetr...
Embodiment 2
[0165] Example 2 Preparation of 2-(3-(9H-purin-6-ylamino)azetidin-1-yl)-5-fluoro-3-phenylquinazolin-4(3H)-one (compound 2)
[0166] Steps 1-4 are the same as in Example 1.
[0167] (5) Preparation of 1-(5-fluoro-4-carbonyl-3-phenyl-3,4-dihydroquinazolin-2-yl)azetidin-3-yl tert-butyl carboxylate
[0168]
[0169] In 50 mL of dichloromethane was added 2-chloro 5-fluoro-3-phenylquinazolin-4(3H)-one (1.4 g, 5.1 mmol), 3-aminoazetidine-1-tert-butyl Carboxylate (0.86g, 5mmol), triethylamine (1.0g, 10mmol), refluxed for 15h, the reaction solution was washed with saturated brine, dried and spin-dried to obtain 0.90g of product, yield: 45%.
[0170] (6) Preparation of 2-(3-aminoazetidin-1-yl)-5-fluoro-3-phenylquinazolin-4(3H)-one
[0171]
[0172] In 50 mL of dichloromethane was added 1-(5-fluoro-4-carbonyl-3-phenyl-3,4-dihydroquinazolin-2-yl)azetidin-3-yl tert-butylcarboxylate Acid ester (0.9g, 2.2mmol), 3mL of trifluoroacetic acid was added under ice-cooling, stirred for...
Embodiment 3
[0178] Example 3 Preparation of 2-(3-(9H-purin-6-ylamino)cyclobutyl)-5-fluoro-3-phenylquinazolin-4(3H)-one (compound 3)
[0179]
[0180] Step 1 is the same as Step 1 in Example 1.
[0181] Step 2 Preparation of 3-((2-fluoro-6-nitrobenzoyl(phenyl)formyl)cyclobutyl tert-butoxycarboxylate
[0182]
[0183] 2-Fluoro-6-nitro-N-phenylbenzamide (13g, 50mmol), DMF (1mL) and thionyl chloride (29.8g, 250mmol) were mixed, stirred at 85°C for 5h, the mixture was spin-dried, and washed with Dichloromethane was dissolved in 100mL, and 3-(tert-butoxycarbonylamino)cyclobutylcarboxylic acid (11.8g, 55mmol) and triethylamine (7.7mL, 55mmol) were added, and the temperature was controlled not to exceed 10°C. Stir at room temperature for 3h, respectively. Wash with water, saturated saline, and protected sodium bicarbonate, dry the organic layer with anhydrous sodium sulfate, and purify by column to obtain 10.5 g of the product, yield: 46%.
[0184] Step 3 Preparation of 3-(5-fluoro-4-c...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap