Doped polyaniline directly-carbonized composite electrocatalyst, preparation method and application
An electrocatalyst, polyaniline technology, applied in physical/chemical process catalysts, chemical instruments and methods, circuits, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]Dissolve 368μL of aniline in 20mL of 1mol / L HCl solution to form a hydrochloric acid solution of aniline, denoted as A; dissolve 1.14g of ammonium persulfate in 20mL of 1mol / L HCl solution, and denote the formed solution as B; then the above Solutions A and B were quickly mixed, and after fully stirring, they were allowed to stand at 20°C for 24 hours to form a paste; add 40mL of acetone to the above paste, stir and filter, and the resulting solid was placed at room temperature for 2 hours. Afterwards, the catalyst precursor was obtained by vacuum drying; the dried catalyst precursor was put into a tube furnace, and N2 was passed through for half an hour to fill the tube furnace with N2, and then the temperature was raised from room temperature to 350°C for 2 hours at a constant temperature. Then the temperature was raised to 700° C. for 2 hours at a constant temperature, and the heating rate was 2° C. / min. Stop passing N2 until it drops to room temperature to obtain the...
Embodiment 2
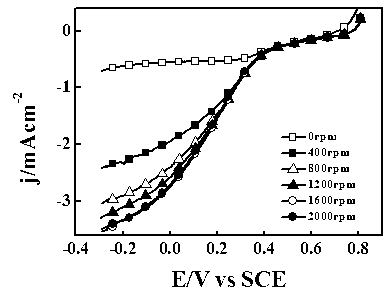
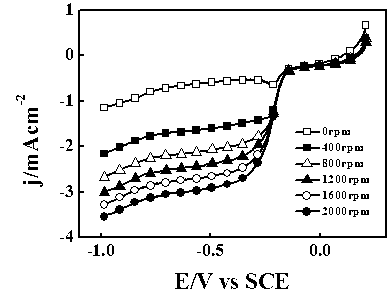
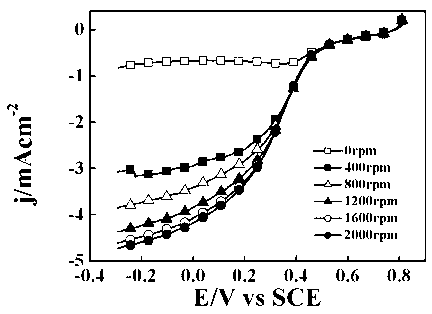
[0033] The preparation process of the composite electrocatalyst for direct carbonization of polyaniline is the same as that of Example 1. The test was carried out in 1 mol / L NaOH aqueous solution saturated with oxygen. The scanning speed is 5 mV / s, and the corresponding linear scanning curve is shown in figure 1 . The onset potential of the oxygen reduction reaction was -0.131 V (vs SCE), and the current density was 2.91mA cm-2-0.5 V (vs SCE) 2000rpm.
Embodiment 3
[0035] Dissolve 368μL of aniline in 20mL of 1mol / L HCl solution to form aniline hydrochloric acid solution, denoted as A; dissolve 1.2mg of FeCl36H2O solid in 20mL of 1mol / L HCl solution, and then add 1.14g of ammonium persulfate to form a solution denoted as It is B; then quickly mix the above solutions A and B, after stirring thoroughly, let stand at 20°C for 24 hours to form a paste; add 80mL of acetone to the above paste, stir and filter to obtain a solid Place it at room temperature for 2 hours, then vacuum dry to obtain the precursor of the catalyst; put the catalyst precursor obtained by drying into a tube furnace, and pass through N for half an hour first. 2 Fill the tube furnace with N 2 , and then the temperature was raised from room temperature to 350° C. for 2 hours, and then raised to 700° C. for 2 hours. The heating rate was 2° C. / min. Stop passing N until it drops to room temperature 2 That is, the direct carbonization product of iron-doped polyaniline is o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com