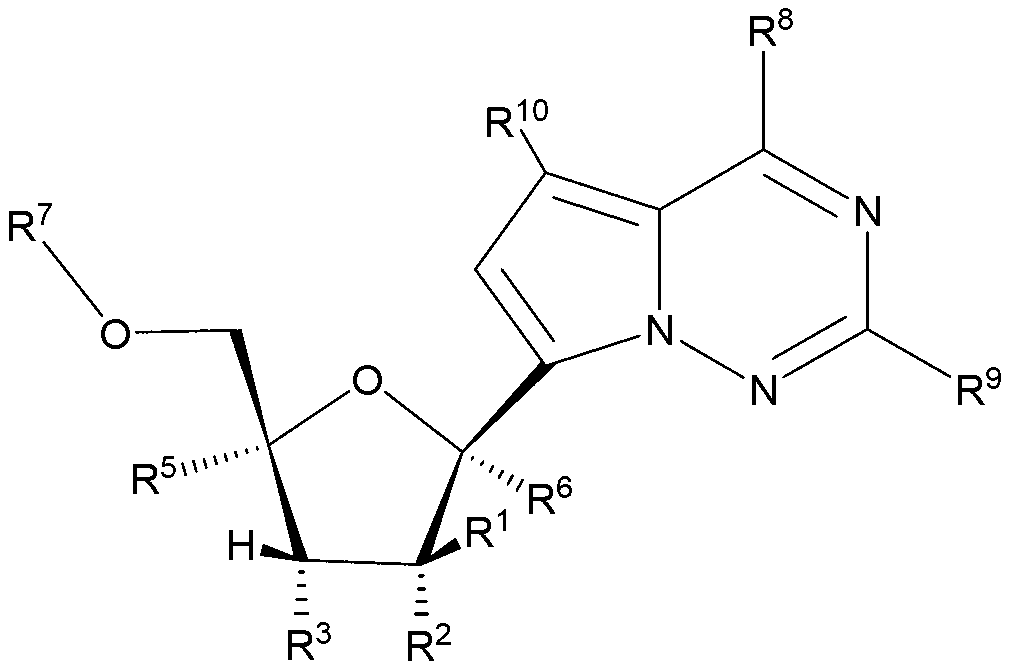
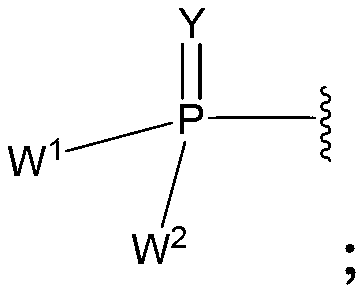
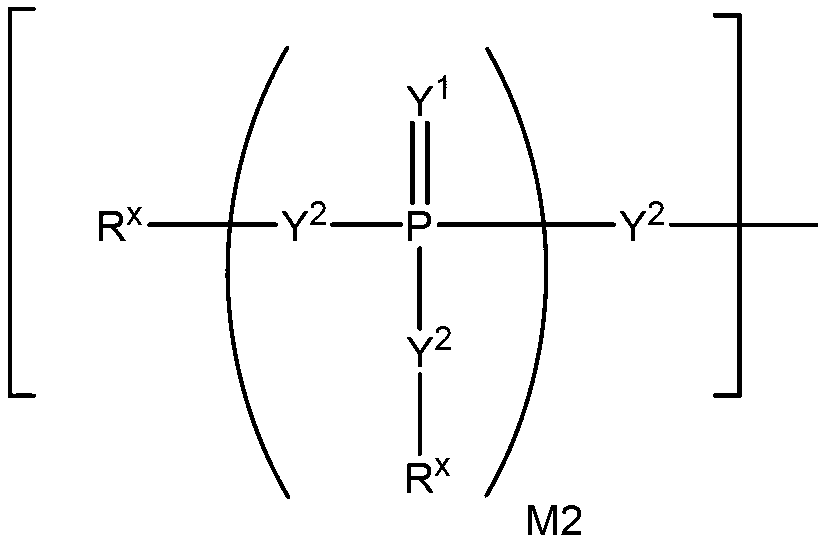
2' -fluoro substituted carba-nucleoside analogs for antiviral treatment
一种C2-C8、正黏病毒的技术,应用在具有抗病毒活性的化合物领域,能够解决未公开正黏病毒科感染有效性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0393] In describing experimental details, certain abbreviations and acronyms are used. Although most of them will be understood by those skilled in the art, Table 1 contains a listing of many such abbreviations and acronyms.
[0394] Table 1. List of abbreviations and acronyms
[0395]
[0396]
[0397]
[0398] Compound preparation
[0399] 2-Deoxy-2-fluoro-4,5-O,O-dibenzyl-D-arabinose
[0400]
[0401] 1'-Methoxy-2-deoxy-2-fluoro-4,5-O,O-dibenzyl-D-arabinose (J.Am Chem.Soc.127( 31), 2005, 10879) (1.0g, 2.88 mmol) with H 2 O (1.5 mL) was treated, and the resulting mixture was stirred for 5 h. The mixture was then diluted with EtOAc (100 mL) and washed with saturated NaHCO 3 (50mL) treatment. The organic layer was separated and washed with NaCl (50 mL), washed over anhydrous MgSO 4 Dry, filter, and concentrate under reduced pressure. The residue was subjected to silica gel chromatography (80 g SiO 2 Combiflash HP Gold Column), eluting with 0-100% EtOAc i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com