Method for inhibiting influenza virus infection and medicament thereof
A technology for influenza virus infection and drugs, applied in the field of treatment of enveloped virus infection, highly pathogenic avian influenza virus and human influenza virus, polypeptide and protein drugs, and treatment of influenza virus, which can solve the problem of interfering with the virus adsorption process, not influenza virus Specific inhibitors, drug-resistant strains, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
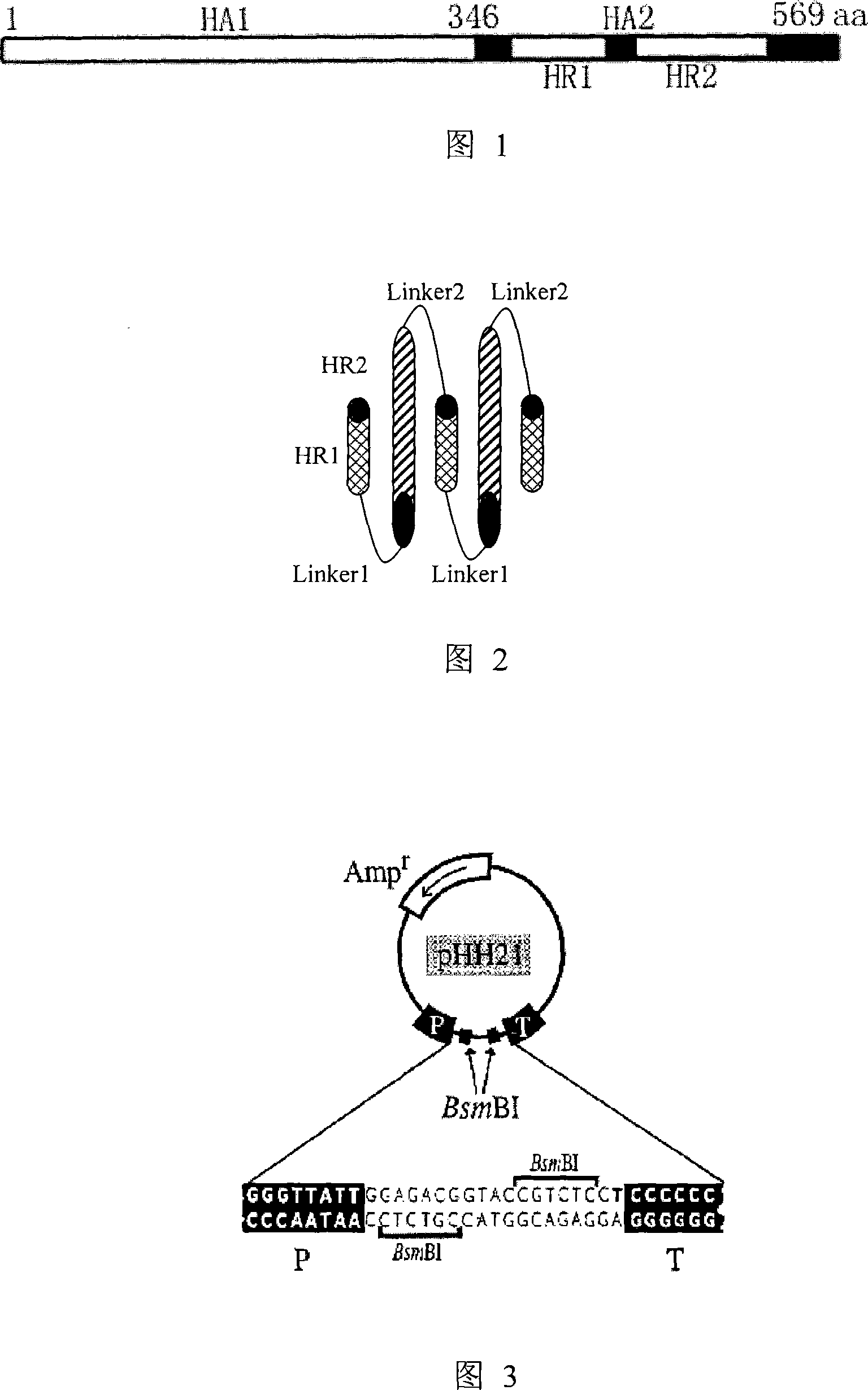
[0055] Embodiment 1: Construction of viral RNA expression plasmid of highly pathogenic avian influenza virus A / bar-headed goose / Qinghai / 1 / 05 strain HA and NA gene
[0056] 1.1) Extraction of viral RNA
[0057] Highly pathogenic avian influenza virus A / bar-headed goose / Qinghai / 1 / 05 strain (hereinafter referred to as QH strain) is a highly pathogenic avian influenza virus of H5N1 subtype, which was isolated and preserved by our laboratory (see literature Liu et al. al, 2005). Centrifuge the chicken embryo allantoic fluid of QH strain influenza virus stored at -70°C at 3000r / m for 10min to remove foreign proteins, then pipette 140μl of the supernatant into a RNase-free 1.5ml centrifuge tube, and use the viral RNA extraction kit (QIAmp Viral RNA Mini Kit, CAT.No.52904) was used to extract viral RNA, and the operation was performed according to the instructions provided by the kit. After extraction, adsorption, washing, centrifugation, and elution, 60 μl of viral RNA solution was...
Embodiment 2
[0073] Embodiment 2: the preparation of recombinant influenza virus WSN and QH-WSN
[0074] 2.1) Preparation of recombinant influenza virus WSN
[0075] The recombinant human influenza virus A / WSN / 33 (being H1N1 subtype, hereinafter referred to as "WSN") used in the experiment was prepared by our laboratory using the influenza virus reverse genetic system (Luytjes et al, 1989) with 12 plasmids. This plasmid reverse genetics system was donated by Professor Yoshihiro Kawaoka from the University of Wisconsin (Neumann et al, 1999). The system includes expression plasmids for expressing eight negative-strand RNA fragments of the influenza virus genome (including pHH21-PB2, pHH21-PB1, pHH21-PA, pHH21-HA, pHH21-NP, pHH21-NA, pHH21-M, pHH21 -NS,) and four protein expression plasmids expressing influenza virus replicase complex (including pcDNA3-PB2, pcDNA3-PB1, pcDNA3-PA, pCAGGS-NP). The above plasmids were transformed into Escherichia coli DH5α competent cells, and the single clone...
Embodiment 3
[0078] Embodiment 3: Plaque titer determination of experimental influenza virus
[0079] In order to detect the inhibitory effect of the polypeptide and protein of the present invention on highly pathogenic avian influenza virus, we detected its inhibitory effect on QH-WSN recombinant virus with H5N1 highly pathogenic avian influenza virus infection characteristics. At the same time, in order to detect whether the above peptides and proteins have cross-inhibitory effects on human influenza viruses, we tested their inhibitory effects on recombinant WSN viruses (human influenza viruses) that have a similar genetic background to QH-WSN but whose surface membrane proteins are H1N1 subtypes ; In addition, we also tested its cross-inhibition effect on the representative strain of human influenza virus H3N2 subtype (A / Jiangxi / 312 / 2005, referred to as "JX" strain) isolated from southern China in 2005 (Blue Rain et al., 2006).
[0080] Since dog kidney passage cells (MDCK) cells are s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com