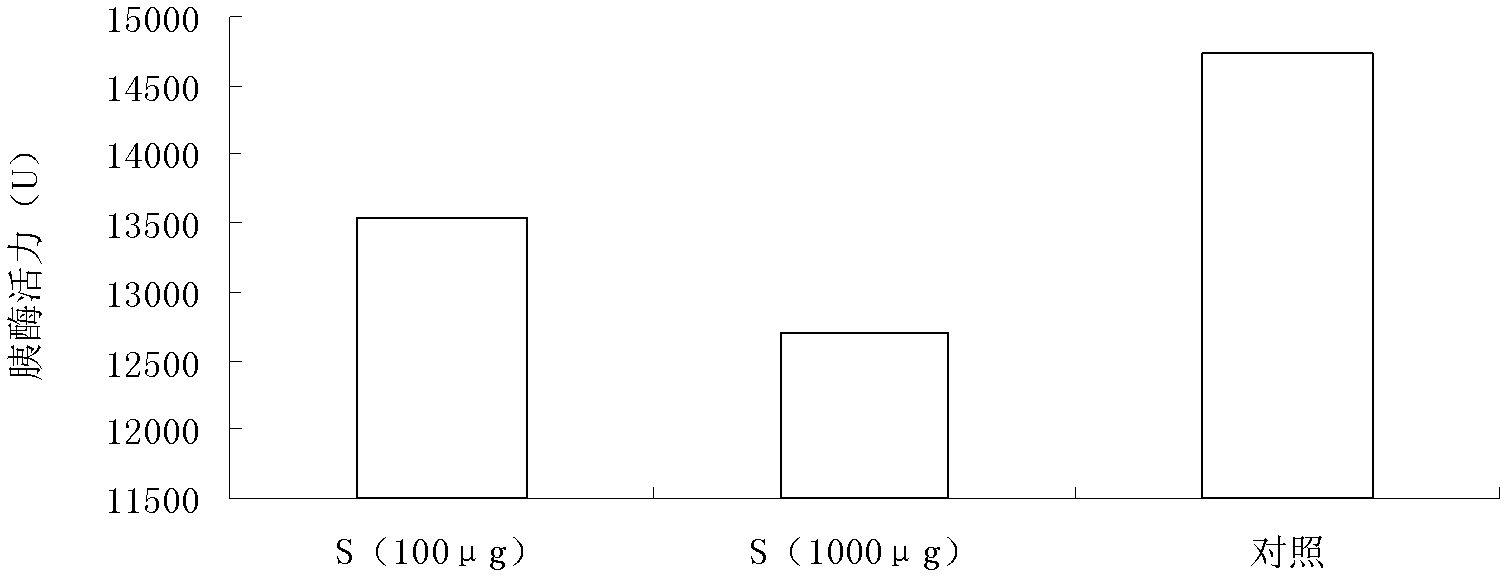Application of Safenour cyclopeptide in oral insulin medicine for treating diabetes
A safenour and diabetes technology, which can be used in cyclic peptide components, drug combinations, and medical preparations containing active ingredients. Hypoglycemic, easy-to-prepare effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] A preparation method of Bacillus amyloliquefaciens WH3 bacterial strain, the steps are:
[0057] 1. Screening and isolation of Bacillus amyloliquefaciens WH3:
[0058] Rapeseed seedlings at the three-leaf stage were randomly selected from the rapeseed field of Hubei Academy of Agricultural Sciences in Wuhan City, Hubei Province, China, and the roots were separated after washing. Rinse the outer surface of rapeseed root with tap water, then rinse it with sterile water for 5 times, then immerse it in 70% (V / V) ethanol for 30 seconds, take it out, rinse it with sterile water for 5 times, and cut it with sterile scissors. The rice roots were cut into pieces about 1 mm in size, and then placed on PDA medium (containing 200 g of potato boiled liquid, 20 g of glucose, 20 g of agar, and distilled water was added to 1 L) and cultured at 28° C. for 48 h. The isolated bacteria were cultured with PD liquid medium (containing 200g potato boiled liquid, 20g glucose, and distilled wa...
Embodiment 2
[0062] Isolation, purification and identification of Safenour
[0063] Inoculate 10 μl of preserved Bacillus amyloliquefaciens WH3 strain into 10 ml sterilized PD medium, and cultivate overnight at 28° C. with shaking at 200 rpm. Then inoculate 2.5ml of fresh culture into a 1L Erlenmeyer flask containing 250ml of fresh PD medium, culture at 28°C or 37°C, and shake at 200rpm for 48h. Generally, 4 Erlenmeyer flasks are used to grow 1 L cultures at a time. Then the supernatant was collected by centrifugation at 8000g for 10min, and the antibacterial active substance was extracted by "two-step extraction method". After alcohol extraction, the n-butanol phase was collected by centrifugation at 8000 g for 20 min. The obtained n-butanol ester phase was evaporated to dryness at 65° C. with a vacuum rotary evaporator, and then dissolved with 10 ml of distilled water. After the solution obtained after dissolution was centrifuged to remove the precipitate, the supernatant was further ...
Embodiment 3
[0065] A kind of application of cyclic peptide Safenour in the oral insulin preparation (medicine) of preparation treatment or prevention diabetes, its steps are:
[0066] 1, a kind of preparation method of Bacillus amyloliquefaciens WH3 bacterial strain, its steps are:
[0067] (1) Screening and isolation of Bacillus amyloliquefaciens WH3:
[0068] Rapeseed seedlings at the three-leaf stage were randomly selected from the rapeseed field of Hubei Academy of Agricultural Sciences in Wuhan City, Hubei Province, China, and the roots were separated after washing. Rinse the outer surface of rapeseed root with tap water, then rinse it with sterile water for 5 times, then immerse it in 70% (V / V) ethanol for 30 seconds, take it out, rinse it with sterile water for 5 times, and cut it with sterile scissors. The rice roots were cut into pieces about 1 mm in size, and then placed on PDA medium (containing 200 g of potato boiled liquid, 20 g of glucose, 20 g of agar, and distilled water ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com